Your Cell's Protein Cleanup Fails With Age—Causing Brain Disease

TL;DR: Scientists have discovered that lipid rafts—tiny organized zones on cell membranes—control critical disease pathways in cancer, Alzheimer's, and infections. New therapies targeting these structures could revolutionize treatment.
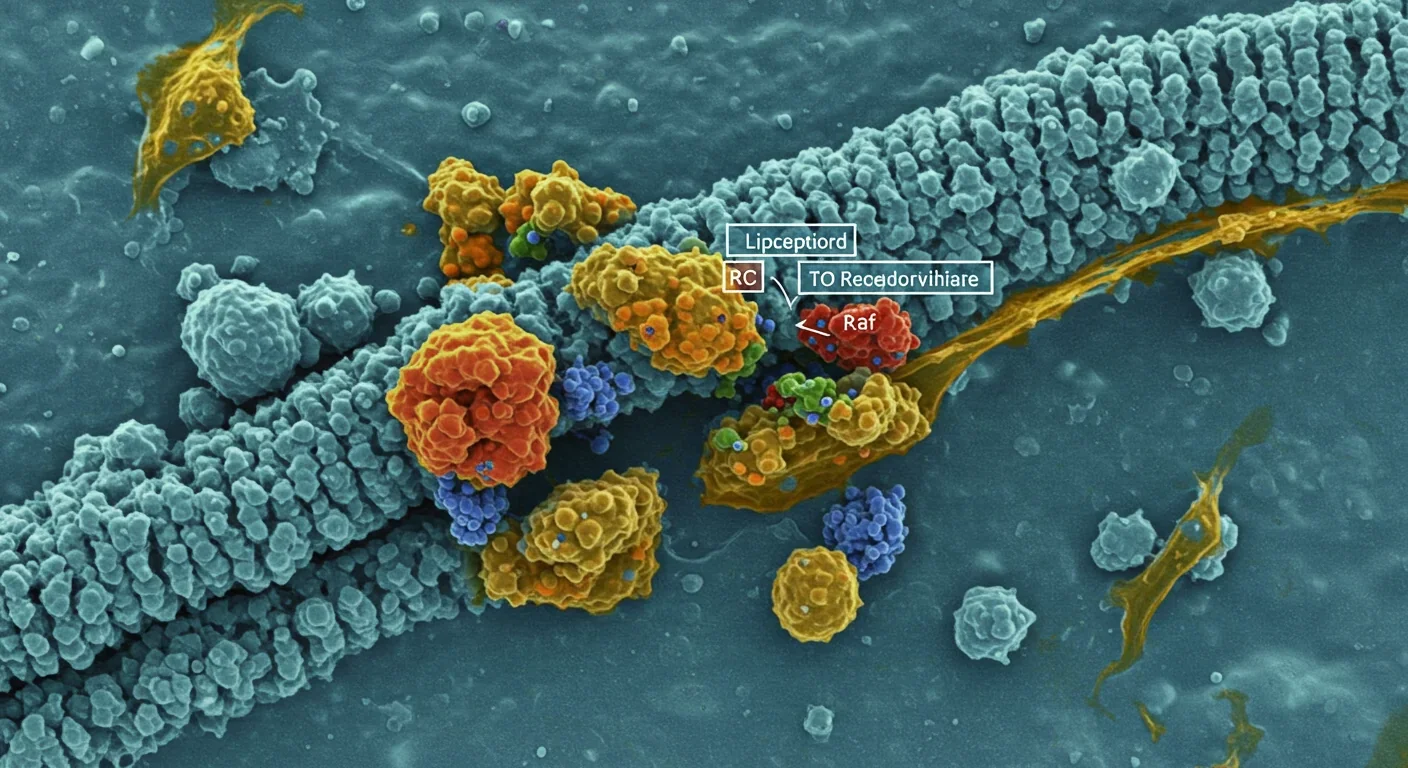
Within every cell in your body, there's an organizational system so subtle that scientists only discovered it a few decades ago. These aren't proteins or genes, but specialized zones on cell membranes called lipid rafts—floating islands of tightly packed molecules that act like coordination hubs for cellular communication. When these tiny structures malfunction, diseases like cancer, Alzheimer's, and viral infections can take hold. Now, researchers are racing to turn this knowledge into breakthrough treatments that could revolutionize how we fight disease.
Think of your cell membrane as a vast ocean. Most of it is fluid and dynamic, with molecules drifting freely. But scattered throughout are specialized microdomains—lipid rafts—that are more like organized ports where specific cargo gets loaded and unloaded. These rafts are enriched with cholesterol and special fats called sphingolipids, making them three to five times denser than the surrounding membrane.
What makes lipid rafts remarkable is their function as organizational platforms. They bring together receptors, signaling proteins, and enzymes in exactly the right configuration to trigger cellular responses. When a hormone or growth factor binds to a receptor sitting in a lipid raft, it doesn't just activate one protein—it orchestrates a cascade involving dozens of molecules that all happen to be clustered in the same neighborhood.
Dr. Maria Sanchez, a leading researcher in the field, describes them as the cellular "docking stations" that bring together receptors, kinases, and adaptor proteins to orchestrate precise signaling cascades. Without these rafts, many of our cells' most critical communications would be slow, imprecise, or impossible.
The cholesterol-rich composition gives rafts their unique properties. The tighter packing of saturated lipids creates a more ordered structure than the surrounding membrane, which allows certain proteins to preferentially associate with rafts while excluding others. This selective partitioning is crucial: it ensures that signaling proteins only interact when they're supposed to, preventing unwanted cellular responses.
The discovery that lipid raft disruption triggers disease pathways has transformed our understanding of multiple conditions. In cancer cells, alterations in raft composition can hyperactivate growth signals while simultaneously helping tumor cells evade the immune system. When researchers deplete cholesterol from cancer cell membranes using a compound called methyl-β-cyclodextrin, they observe something remarkable: the cancer cells' growth signals collapse because the signaling proteins can no longer cluster together properly.
Neurodegenerative diseases reveal an even more intricate relationship with lipid rafts. In Alzheimer's disease, altered raft composition actively promotes the production of toxic amyloid-beta plaques. Patients with familial Alzheimer's mutations exhibit changes in the glycolipid content of their rafts, creating an environment that accelerates plaque formation. The rafts essentially become factories for the very proteins that destroy neurons.
Perhaps most surprising is the role of lipid rafts in infectious disease. Viruses are opportunistic, and many have evolved to exploit lipid rafts as entry points into cells. Influenza, HIV, Ebola, and even SARS-CoV-2 all target raft-associated receptors to gain access to cells. The influenza virus hemagglutinin protein specifically binds to GM1 gangliosides concentrated in rafts. By hijacking these organized platforms, viruses can rapidly mobilize the cellular machinery they need for replication.
Bacterial pathogens also target rafts. Cholera toxin, for instance, binds to GM1 gangliosides in rafts before being internalized, where it wreaks havoc on cellular signaling. The pattern is consistent: pathogens have evolved to increase infectivity and evade immune defenses by targeting the cell's most critical organizational hubs.
The metabolic diseases tied to raft dysfunction are equally striking. Insulin resistance, the hallmark of type 2 diabetes, involves disruptions in how insulin receptors cluster within lipid rafts. When raft dynamics are impaired—often through oxidative stress and inflammatory signaling—the insulin receptor can't efficiently trigger glucose uptake. The result is elevated blood sugar and the cascade of complications that follow.
The concept of lipid rafts emerged in the late 1990s as researchers began questioning the prevailing "fluid mosaic model" of cell membranes. That model, proposed in 1972, depicted membranes as uniform seas of lipids with proteins floating freely. But accumulating evidence suggested otherwise. Scientists noticed that certain membrane proteins resisted solubilization by detergents, hinting at more ordered structures.
The breakthrough came when researchers isolated these detergent-resistant membrane fractions and analyzed their composition. They found cholesterol and sphingolipid-rich domains that functioned as distinct organizational units. The term "lipid raft" captured the idea: these were dynamic, floating platforms that could move through the membrane while maintaining their internal organization.
Early skepticism focused on whether rafts were real biological structures or artifacts of the isolation process. That debate persisted for years, but advances in microscopy—particularly super-resolution imaging techniques—finally visualized rafts in living cells. These images confirmed that rafts are genuine cellular features, typically 10-200 nanometers in diameter, that coalesce and disperse based on the cell's needs.
This discovery paralleled other shifts in cell biology. Just as we learned that genomes aren't simply linear sequences but three-dimensional structures with functional territories, we discovered that membranes aren't homogeneous but organized into specialized zones. Both insights revealed that biology operates through spatial organization at scales we could barely measure.

Now comes the exciting part: translating this basic science into medicine. One of the most promising approaches is engineered lipid raft transplantation. Researchers are developing synthetic lipid mixtures that can replace or supplement dysfunctional rafts in diseased cells. In obese mouse models with insulin resistance, transplanting engineered rafts restored insulin sensitivity by recreating the proper signaling environment.
Another strategy involves targeted disruption of pathological rafts. In cancer, where certain rafts promote tumor growth, researchers are designing nanobodies—tiny antibody fragments—that bind to specific gangliosides like GM3 concentrated in tumor rafts. These nanobodies destabilize the rafts, impairing integrin clustering that cancer cells need for metastasis. Early results in melanoma models show reduced spread of the disease.
For neurodegenerative conditions, the approach focuses on modulating cholesterol within rafts. Statins, widely used to lower blood cholesterol, also affect membrane cholesterol and may reduce amyloid-beta production by altering the composition of neuronal rafts. More targeted drugs are in development that can selectively modify raft composition in the brain without affecting cholesterol throughout the body.
In infectious disease, blocking viral entry through raft disruption offers a novel antiviral strategy. Researchers have developed cholesterol analogs that can be pre-administered to cells, making rafts inhospitable to viral attachment proteins. In one study, n-alkyl cholesterol analogs blocked influenza A virus entry without harming cell viability, suggesting a potential prophylactic approach for respiratory infections.
Mass spectrometry has revolutionized our ability to characterize raft composition with precision. By identifying exactly which lipids and proteins reside in rafts under healthy and diseased conditions, scientists can pinpoint therapeutic targets. This technology allows researchers to track how experimental drugs alter raft structure, providing real-time feedback for drug development.
What makes raft-targeted therapies so compelling is their precision. Traditional drugs often act broadly, affecting entire classes of molecules throughout the body. But by targeting the spatial organization of signaling complexes, raft-based approaches can modulate specific pathways while leaving others intact.
Consider the implications for cancer treatment. Current therapies targeting growth factor receptors like EGFR can cause severe side effects because these receptors exist throughout the body. But if we could selectively disrupt the rafts where EGFR signaling occurs in tumors, we might achieve cancer suppression with fewer systemic effects. Early research suggests this selectivity is achievable, because tumor cells often have altered raft compositions that distinguish them from healthy tissue.
The potential extends to personalized medicine. Since raft composition varies between individuals and changes with disease progression, analyzing a patient's membrane lipid profile could guide treatment selection. Someone with Alzheimer's who shows specific changes in neuronal raft glycolipids might benefit from a different intervention than someone with a different lipid signature.
There's also promise in combination approaches. Pairing raft-modulating drugs with conventional therapies could enhance effectiveness. For instance, temporarily disrupting rafts in cancer cells might make them more vulnerable to chemotherapy by interfering with drug resistance mechanisms that rely on raft-mediated signaling.
Despite the excitement, significant hurdles remain. Lipid rafts are dynamic structures that form and dissolve in milliseconds, making them difficult to target with conventional drugs. Developing molecules that can selectively interact with these transient platforms requires sophisticated chemical engineering.
Another challenge is delivery. Getting raft-modulating compounds to the right cells—whether in the brain, a tumor, or specific immune cells—requires advanced drug delivery systems. Nanoparticles and liposomes show promise, but ensuring they reach their target without triggering immune responses or toxicity remains difficult.
The heterogeneity of rafts complicates matters further. Not all rafts are alike; they vary in composition depending on cell type, location within the membrane, and cellular state. A therapy that disrupts one type of raft might have no effect—or unintended consequences—on others. Understanding this diversity is crucial for designing safe, effective interventions.
Regulatory pathways for raft-targeted therapies are also unclear. How do you validate that a drug actually modulates raft function in patients? Standard clinical trial endpoints may not capture the nuanced changes in membrane organization that these therapies aim to achieve. New biomarkers and imaging techniques will be needed to demonstrate efficacy.
Research on lipid rafts is happening worldwide, with different regions bringing unique perspectives and strengths. Asian laboratories, particularly in Japan and South Korea, have excelled in the high-resolution imaging techniques that first visualized rafts in living cells. Their work continues to push the boundaries of what we can see at the nanoscale.
European researchers have led much of the therapeutic development, with groups in Germany and Switzerland pioneering raft-modulating compounds. The Netherlands has become a hub for computational modeling of raft dynamics, helping predict how interventions might affect membrane organization before expensive experiments begin.
In the United States, the focus has been on translating basic discoveries into clinical applications, with several biotech companies now working on raft-targeted cancer therapies. The National Institutes of Health has designated lipid raft research as a priority area, recognizing its potential impact across multiple diseases.
Collaboration is essential because the complexity of raft biology exceeds what any single lab or nation can tackle alone. International consortia are sharing data on raft composition across different diseases, building comprehensive databases that reveal patterns invisible to individual studies. This global approach accelerates progress by allowing researchers to build on each other's findings rather than duplicating efforts.
For patients, the potential impact of raft-targeted therapies could be transformative. Imagine a future where Alzheimer's progression could be slowed by a drug that simply reorganizes the lipid content of brain cell membranes, reducing toxic protein production without the severe side effects of current experimental treatments.
Cancer patients might receive therapies that disrupt tumor cell signaling while sparing healthy tissues, leading to better outcomes with less suffering. The immunotherapy revolution could be enhanced by manipulating immune cell rafts to boost anti-tumor responses or dampen autoimmune reactions.
In infectious disease, raft-blocking compounds might offer a new line of defense against emerging viral threats. Unlike traditional antivirals that target specific viral proteins and can become ineffective through mutation, blocking the host cell structures that viruses depend on could provide broader, more durable protection.
Within the next decade, it's plausible that lipid raft analysis will become part of routine diagnostics. A simple blood test revealing your membrane lipid profile could flag disease risk years before symptoms appear, enabling preventive interventions. This shift from reactive to proactive medicine represents one of healthcare's most promising frontiers.
As raft-targeted therapies move from laboratory to clinic, understanding this biology becomes increasingly relevant. For patients facing diseases linked to raft dysfunction, staying informed about emerging treatments could open new options when conventional approaches fail.
Healthcare providers will need education on this dimension of cellular biology. Just as doctors learned to consider genetic factors in treatment decisions, they'll need to think about membrane organization and how therapies might affect it. Medical schools are beginning to incorporate raft biology into curricula, recognizing its growing clinical importance.
For the broader public, the lipid raft story illustrates how fundamental research—the kind that seeks to understand basic biological mechanisms without immediate applications in mind—eventually yields practical benefits. The scientists who first characterized these membrane structures in the 1990s couldn't have imagined they were laying groundwork for treatments that might help millions. It's a reminder that supporting curiosity-driven science is an investment in future solutions to problems we haven't yet identified.
The lipid raft revolution also highlights the importance of interdisciplinary science. Progress required insights from biochemistry, cell biology, physics, engineering, and medicine working in concert. As we face increasingly complex health challenges, this collaborative approach will be essential.
The journey from discovering lipid rafts to developing effective therapies based on them exemplifies both the promise and the patience required for medical progress. It took decades to move from initial observations to where we are today: multiple clinical trials testing raft-targeted interventions for various diseases.
Several Phase I and II trials are evaluating cholesterol-modulating compounds specifically designed to alter raft composition in cancer and neurodegenerative diseases. While results are preliminary, early safety data are encouraging. The real test will come in larger trials determining whether raft modulation produces meaningful clinical benefits.
Regulatory agencies are watching closely. The FDA and European Medicines Agency have begun consultations with researchers developing raft-targeted therapies, working to establish appropriate endpoints and safety standards. This proactive approach could accelerate approval once definitive efficacy data emerge.
The commercial landscape is evolving too. Pharmaceutical giants are partnering with biotech startups focused on raft biology, bringing resources and expertise in drug development. This influx of investment reflects growing confidence that raft-targeted therapies will become a significant therapeutic modality.
What happens in laboratories today will shape medicine tomorrow. Each discovery about how rafts function, how they fail in disease, and how we might intervene adds another piece to a puzzle that could transform healthcare. The invisible gatekeepers of cellular communication are finally revealing their secrets—and those secrets may hold keys to treating some of humanity's most devastating diseases.

Curiosity rover detects mysterious methane spikes on Mars that vanish within hours, defying atmospheric models. Scientists debate whether the source is hidden microbial life or geological processes, while new research reveals UV-activated dust rapidly destroys the gas.

CMA is a selective cellular cleanup system that targets damaged proteins for degradation. As we age, CMA declines—leading to toxic protein accumulation and neurodegeneration. Scientists are developing therapies to restore CMA function and potentially prevent brain diseases.

Intercropping boosts farm yields by 20-50% by growing multiple crops together, using complementary resource use, nitrogen fixation, and pest suppression to build resilience against climate shocks while reducing costs.

The Baader-Meinhof phenomenon explains why newly learned information suddenly seems everywhere. This frequency illusion results from selective attention and confirmation bias—adaptive evolutionary mechanisms now amplified by social media algorithms.

Plants and soil microbes form powerful partnerships that can clean contaminated soil at a fraction of traditional costs. These phytoremediation networks use biological processes to extract, degrade, or stabilize toxic pollutants, offering a sustainable alternative to excavation for brownfields and agricultural land.

Renters pay mortgage-equivalent amounts but build zero wealth, creating a 40x wealth gap with homeowners. Institutional investors have transformed housing into a wealth extraction mechanism where working families transfer $720,000+ over 30 years while property owners accumulate equity and generational wealth.
AlphaGo revolutionized AI by defeating world champion Lee Sedol through reinforcement learning and neural networks. Its successor, AlphaGo Zero, learned purely through self-play, discovering strategies superior to millennia of human knowledge—opening new frontiers in AI applications across healthcare, robotics, and optimization.