Epigenetic Clocks Predict Disease 30 Years Early

TL;DR: Scientists discovered that extracellular vesicles (EVs)—nano-sized packages released by all cells—are both cancer's secret weapon for metastasis and stem cells' healing tool. Cancer hijacks EVs to prime distant organs, suppress immunity, and transfer drug resistance, while stem-cell EVs repair hearts, regenerate cartilage, and modulate inflammation without transplanting cells. Over 200 clinical trials are engineering EVs as programmable drug carriers and liquid biopsy biomarkers. Mastering this cellular postal service could revolutionize how we diagnose and treat disease—if we solve scalability, standardization, and regulatory hurdles first.

Scientists have discovered that the same microscopic packages our cells use to heal wounds are also the secret weapons cancer uses to spread through the body. These tiny bubbles—extracellular vesicles, or EVs—are rewriting everything we thought we knew about how diseases progress and how healing happens. In 2025, researchers identified EVs as both the villain that primes distant organs for metastasis and the hero that delivers stem-cell therapy without the stem cells. This biological double agent is now the hottest target in medicine, with over 200 clinical trials underway and the first EV-based therapies entering hospitals.
The stakes are enormous: if we can hijack this cellular mail system, we might deliver drugs directly to tumors, regenerate damaged hearts, and diagnose cancer from a single drop of blood. But if we misunderstand it, we could accelerate the very diseases we're trying to stop.
For decades, biologists assumed cells communicated mainly through direct contact or by releasing soluble molecules that drift through bodily fluids. Then came the revelation: cells were also sending packages. Every cell type—neurons, immune cells, cancer cells, stem cells—constantly releases membrane-bound nanoparticles ranging from 30 to 1,000 nanometers in diameter. These extracellular vesicles carry proteins, lipids, DNA fragments, and especially RNA molecules that can reprogram recipient cells hundreds of miles away from their source.
Think of EVs as cellular flash drives. A cancer cell in the breast can send EVs to the liver, delivering instructions that remodel liver tissue to create a welcoming environment for future metastatic colonization—a "pre-metastatic niche." Meanwhile, a mesenchymal stem cell in bone marrow can dispatch EVs loaded with anti-inflammatory signals that calm an overactive immune system and promote tissue repair after a heart attack.
The cargo selection process is exquisitely precise. Inside cells, multivesicular bodies—compartments that look like Swiss cheese under an electron microscope—bud inward to form intraluminal vesicles. These are loaded with specific proteins, lipids, and nucleic acids through several pathways. The ESCRT machinery (Endosomal Sorting Complex Required for Transport) acts like a molecular conveyor belt, recognizing ubiquitin tags on proteins and shepherding them into budding vesicles. Tetraspanins—proteins that span the membrane four times—create lipid raft platforms enriched in cholesterol and sphingolipids, clustering cargo molecules before they're packaged. RNA-binding proteins such as hnRNPA2B1 and YBX1 recognize specific sequence motifs on microRNAs, determining which genetic messages get exported and which stay behind.
This selective sorting means EVs aren't random cellular debris—they're curated messages. When a multivesicular body fuses with the plasma membrane, it releases its contents as exosomes. Alternatively, the plasma membrane can bleb outward directly, pinching off microvesicles. The result is a constant stream of information-rich nanoparticles entering the bloodstream, lymph, cerebrospinal fluid, and even saliva.
Cancer cells are master manipulators of the EV system. Tumor-derived EVs are molecular Swiss Army knives of metastasis, wielding multiple mechanisms to prepare the body for invasion.
Priming Distant Organs: Breast cancer cells release EVs enriched with integrin proteins—specifically integrin αvβ5—that bind to liver cells. Once docked, these EVs trigger inflammatory signals and recruit bone-marrow-derived macrophages, transforming the liver into a hospitable landing pad for circulating tumor cells. Pancreatic cancer microvesicles use CD36 receptors to penetrate liver tissue, activating Kupffer cells (liver macrophages) to secrete factors that promote tumor growth. Exosomal miR-21 from breast cancer drives osteoclast formation in bone, hollowing out niches where metastatic cells later lodge. This organ-specific targeting—termed organotropism—explains why certain cancers preferentially metastasize to lungs, liver, or bone.
Immune Suppression: Tumor EVs carry immune checkpoint proteins, especially PD-L1, on their surface. When these vesicles encounter cytotoxic T cells, PD-L1 binds to the PD-1 receptor, delivering a "stand down" signal that exhausts the immune attack. Exosomal TGF-β and FasL amplify this effect, expanding regulatory T cells that actively suppress anti-tumor immunity. In melanoma models, platelet-derived EVs shield circulating tumor cells from natural killer cells by expressing GITRL, a decoy ligand that prevents immune clearance. The result: tumors create a systemic immunosuppressive environment that extends far beyond the primary mass.
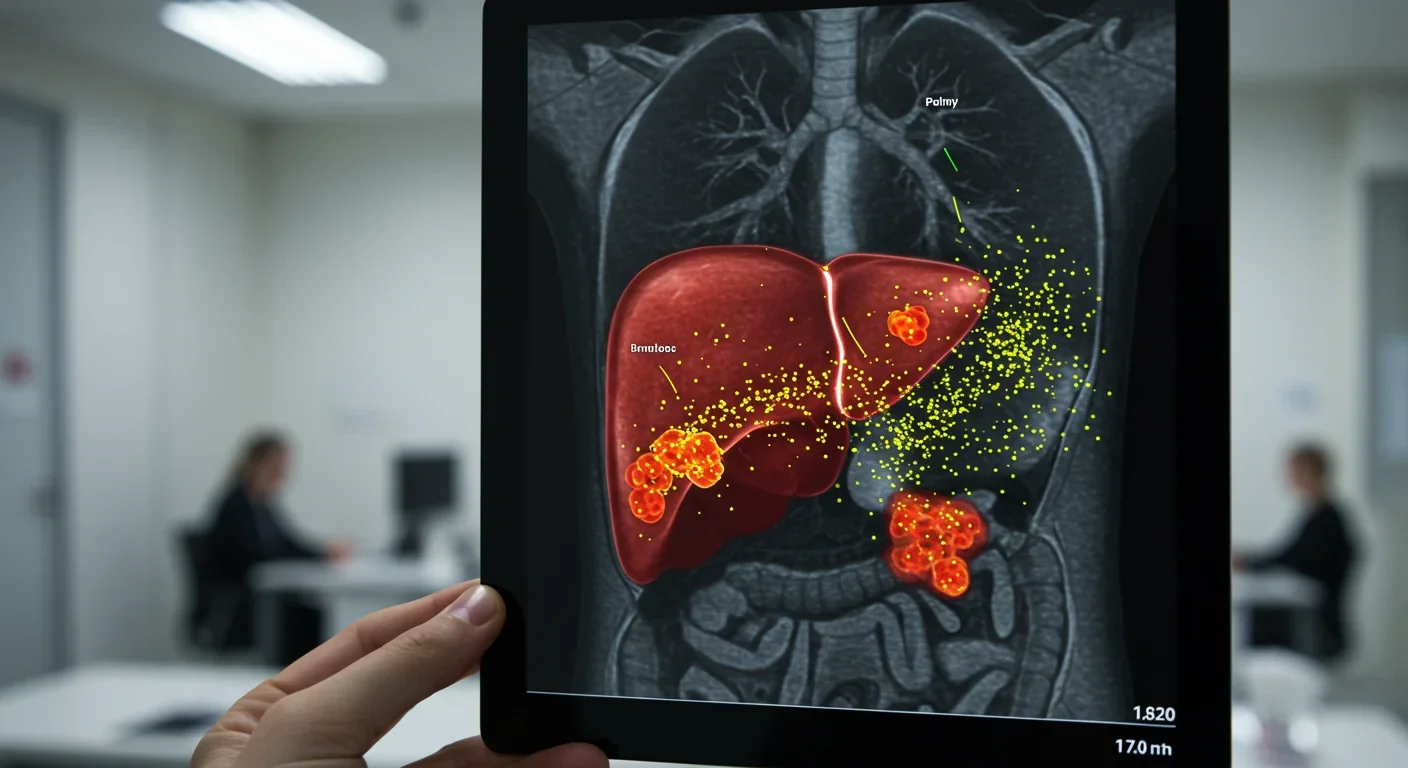
Drug Resistance Transfer: Cancer cells that survive chemotherapy don't keep their survival secrets to themselves. Drug-resistant cells release EVs packed with efflux pumps—proteins like P-glycoprotein and BCRP that eject toxins from cells. When sensitive cancer cells internalize these EVs, they inherit functional resistance machinery within hours. Glioblastoma EVs deliver P-glycoprotein that confers three-fold resistance to temozolomide. Breast cancer exosomes transfer miR-21 and miR-155, microRNAs that suppress apoptosis and promote survival pathways in recipient cells. This horizontal gene transfer allows tumors to share survival strategies faster than Darwinian selection alone.
Angiogenesis and Matrix Remodeling: Hypoxic tumor regions—starved of oxygen—ramp up EV production. These hypoxia-induced vesicles carry VEGF, HIF-1α, and matrix metalloproteinases that stimulate blood vessel growth and dissolve extracellular barriers, paving highways for invasion. Exosomal circPSMA1 from triple-negative breast cancer acts as a microRNA sponge, activating the Akt1/β-catenin pathway to drive metastasis and immune evasion.
If tumor EVs are weapons, stem-cell EVs are medics. Mesenchymal stem cells (MSCs)—multipotent cells found in bone marrow, fat, and umbilical cord—have long been used in regenerative medicine. But MSC transplants come with risks: limited survival, tumor formation potential, and immune rejection. Enter MSC-derived exosomes: they deliver the therapeutic punch without the cells.
Cardiac Repair: After a heart attack, scar tissue replaces dead muscle, weakening the heart. MSC exosomes injected into infarcted myocardium increase Akt phosphorylation, a survival signal that reduces oxidative stress and cell death. They deliver pro-angiogenic factors like VEGF and HIF-1α, sprouting new blood vessels to feed surviving tissue. In a mouse model, a fibrinogen-MSC-exosome hydrogel sprayed onto damaged hearts formed a protective film that accelerated angiogenesis and improved cardiac function by 30% within 12 weeks. A Phase I/II trial (NCT04223478) in acute myocardial infarction patients showed that adipose-derived MSC exosomes improved left ventricular ejection fraction with no serious adverse events.
Cartilage and Bone Regeneration: Osteoarthritis patients suffer from chronic inflammation and cartilage degradation. MSC exosomes suppress pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and matrix-degrading enzymes (MMP-13, ADAMTS-5) while upregulating collagen II synthesis. They polarize macrophages from a pro-inflammatory M1 state to an anti-inflammatory M2 state via TGF-β and IL-10 signaling. Intra-articular injection of human umbilical cord MSC exosomes in rats with osteoarthritis led to complete cartilage regeneration by 12 weeks. MSC exosomes enriched with miR-21-5p and miR-210 stimulate osteoblast differentiation via the PI3K/AKT pathway, accelerating bone defect healing.
Neurological and Renal Repair: MSC exosomes cross the blood-brain barrier when functionalized with targeting peptides, delivering neuroprotective microRNAs that reduce inflammation in stroke and Alzheimer's models. In acute kidney injury, exosomes activate the ERK1/2 pathway, inhibiting apoptosis and promoting tubular cell proliferation, lowering kidney injury scores by 40% in preclinical studies.
Advantages Over Cell Therapy: Exosomes are acellular, so they can't form tumors or trigger graft-versus-host disease. They're easier to manufacture, store, and dose. They bypass the complex logistics of live-cell handling and can be freeze-dried for long-term stability. Critically, they maintain the therapeutic effects of MSCs—angiogenesis, anti-inflammation, matrix remodeling—without the risks.
Traditional cancer diagnostics rely on imaging and tissue biopsies—invasive, slow, and prone to missing micrometastases. EVs offer a real-time snapshot of disease from a blood draw.
Tumor-Educated Platelets: Platelets circulating through a tumor uptake tumor-derived EVs and RNAs, becoming "tumor-educated platelets" (TEPs). Their mRNA and microRNA profiles reflect the tumor's molecular state. TEPs can distinguish cancer patients from healthy individuals with over 90% accuracy and even identify the tumor type—lung, breast, glioblastoma—based on their RNA signatures. Platelet-derived EV PD-L1 levels predict response to checkpoint inhibitors in non-small cell lung cancer, offering a way to stratify patients before expensive immunotherapy.
Circulating Exosomal Biomarkers: Urinary exosomes enriched for specific microRNAs correlate with prostate cancer progression. Salivary exosomes carrying oncogenic protein signatures enable early detection of oral cancers with diagnostic accuracy comparable to tissue biopsies. In a Phase II study, exosomal integrin profiles from colorectal cancer patients predicted liver-specific metastasis, allowing preemptive monitoring.
Advantages: Liquid biopsies are non-invasive, repeatable, and capture tumor heterogeneity across multiple metastatic sites. EVs remain stable in stored plasma for up to 90 days, enabling retrospective analysis. Limitations include the need for standardized isolation methods, variability due to pre-analytical handling (platelet activation during blood draw can skew results), and the requirement for large validation cohorts to establish sensitivity and specificity thresholds.

The next frontier is turning EVs from passive messengers into programmable drug delivery vehicles.
Targeting Strategies: Native EVs distribute widely, often accumulating in liver and spleen. To achieve precision, researchers genetically engineer donor cells to display targeting ligands on exosome surfaces. Fusing the RVG29 peptide to Lamp2b—a transmembrane exosomal protein—directs EVs to neurons via nicotinic acetylcholine receptors, enabling delivery of siRNA across the blood-brain barrier with >60% target knockdown in Alzheimer's mouse models. Integrin-rich exosomes can be decorated with RGD peptides or antibody fragments to bind αvβ3-positive tumor cells, achieving three-fold higher uptake than unmodified vesicles.
Cargo Loading: Passive incubation allows modest loading (10-30%) of siRNA or microRNA via electrostatic attraction. Active methods—electroporation, sonication, or detergent permeabilization—force nucleic acids or small molecules into the lumen, achieving up to 70% loading but risking vesicle aggregation. Hybrid exosome-liposome nanovesicles combine natural surface markers for receptor engagement with synthetic lipid layers for high drug loading (>10 µg/mL), preserving both targeting and payload capacity. Genetically engineering donor cells to overexpress therapeutic RNA or proteins ensures endogenous packaging during biogenesis, yielding more homogeneous preparations.
Surface Modifications: PEGylation—coating exosomes with polyethylene glycol—reduces hepatic clearance by 80%, extending circulation time beyond 24 hours. Modulating surface charge directs biodistribution: anionic exosomes preferentially accumulate in liver, while zwitterionic ones target kidneys, offering passive organ-specific delivery. Click chemistry enables conjugation of aptamers or UV-triggered release molecules, allowing spatiotemporal control.
Clinical Applications: In a Phase I/II trial (NCT04911234), 28 metastatic breast cancer patients received exosome-encapsulated paclitaxel (EV-PTX); 10 achieved partial remission with a median progression-free survival of 6.5 months. MSC-derived exosomes loaded with BMP-7 attenuated osteoarthritis in rat models by promoting M2 macrophage polarization. Plant-derived exosomes from ginger and celery, orally bioavailable and capable of penetrating the gut epithelium, are being tested as edible nanocarriers for anti-cancer alkaloids.
Blocking Pathogenic EVs: Pharmacological inhibition of neutral sphingomyelinase 2 (nSMase2) with GW4869 reduces exosome secretion by 60% in preclinical tumor models, cutting bone osteoclast activity by 45% and slowing metastasis. Immunoadsorption devices like the pro-metastatic derivative eliminator (PMDE) filter circulating tumor cells and tumor exosomes from blood, reducing metastatic burden in mice. Selective disruption of ESCRT components or tetraspanin platforms could block tumor EV production while sparing physiological vesicles.
Despite promise, EV therapeutics face formidable obstacles before becoming standard care.
Standardization: EV isolation methods—differential ultracentrifugation, density gradients, size-exclusion chromatography, polymer precipitation, immunoaffinity capture—yield different purity and activity profiles. Ultracentrifugation co-isolates lipoproteins and protein aggregates; polyethylene glycol precipitation leaves polymer contaminants. The International Society for Extracellular Vesicles' MISEV2023 guidelines mandate reporting isolation method, instrument detection limits, and pre-analytical variables, yet inter-laboratory reproducibility remains a challenge.
Scalability: Producing therapeutic-grade exosomes at clinical scale is arduous. MSCs secrete only ~10^9 exosomes per liter of culture medium. To treat a single patient requires liters of conditioned media processed through multi-step purification. Tangential flow filtration and continuous bioreactors are improving yields, but batch-to-batch variability in cargo composition complicates quality control.
Potency Assays: Unlike small-molecule drugs with defined structures, EVs are heterogeneous mixtures. Regulatory agencies (FDA, EMA) require assays that measure not just particle count but functional potency—e.g., the ability of cardiac exosomes to reduce infarct size or of immunomodulatory exosomes to suppress T-cell proliferation. Current assays lack standardization.
Regulatory Pathways: EVs occupy a gray zone: are they biologics, cell therapies, or gene therapies? The EMA's MIRACUM EV working group and FDA guidance documents (2024) classify EV-based products as biological nanomedicines, requiring demonstration of safety, biodistribution, pharmacokinetics, and efficacy similar to other biologics. No EV diagnostic or therapeutic has yet received full FDA approval, though several are in Phase II trials.
Shelf Life: Stability data for EV formulations are sparse. Freeze-drying (lyophilization) extends storage but may alter surface proteins. No published studies define shelf-life under pharmaceutical storage conditions, a prerequisite for commercialization.
The field is exploding with new discoveries that could unlock next-generation therapies.
Nuclear-Derived EVs: Researchers identified vesicles budding directly from the nucleus (nEVs) carrying full-length chromosomal DNA fragments. Genotoxic drugs increase nEV release, suggesting cells use them to jettison damaged DNA. Could nEVs serve as real-time monitors of genomic instability?
Exomeres: Particles smaller than 35 nm—exomeres—have distinct lipid and protein compositions compared to classical exosomes. Their roles remain mysterious, but their ultrasmall size might enable deeper tissue penetration.
Platelet-Derived EV Signatures: Different platelet activators (collagen, snake venom) produce EVs with distinct transcriptional effects. Collagen-activated platelet EVs upregulate TGF-β signaling in melanoma, while fibrin-activated ones boost interferon responses. This pathway-specific reprogramming suggests that antiplatelet drugs might need to be tailored to block specific EV subsets.
Tumor-Free Organ EVs: Paradoxically, EVs from distant healthy organs in tumor-bearing mice promote metastasis to those organs. Brain-derived EVs from mice with breast tumors elsewhere contain metastasis-related proteins and induce brain metastasis when injected with tumor cells, revealing that pre-metastatic remodeling extends beyond tumor-derived EVs.
CRISPR Delivery: MSC exosomes co-loaded with Cas9 protein and guide RNAs are being tested for in situ gene correction in muscular dystrophy models, potentially offering a safer alternative to viral vectors.
Extracellular vesicles are biological couriers with immense power: they can carry death sentences or healing instructions, depending on their source and cargo. Cancer exploits them to colonize distant organs, suppress immunity, and spread drug resistance. Stem cells harness them to regenerate tissues, calm inflammation, and restore function—without the risks of cell transplantation.
The next decade will determine whether we can turn this cellular postal service into a precision medicine platform. Early clinical trials show that EV-based therapies can deliver drugs to hard-to-reach tumors, diagnose cancers from blood, and repair damaged organs. But translating bench discoveries into bedside treatments requires solving scalability, standardization, and regulatory puzzles.
For patients, the promise is tantalizing: a world where heart attack damage is reversed by injected nanoparticles, where cancer is detected years earlier from a blood test, and where chemotherapy is delivered only to tumor cells, sparing healthy tissue. For scientists, the challenge is to decode the molecular grammar of EV cargo selection, master the engineering of designer vesicles, and understand when blocking EVs helps and when it harms.
The biology is clear: every cell in your body is constantly sending messages in bottles. Whether those messages heal or harm depends on who's sending them—and whether we're smart enough to intercept, reprogram, or amplify them. The cellular postal service is running 24/7. The question is: can we become postmasters?
This is not a distant dream. Over 200 clinical trials are underway. The first EV-based diagnostics are entering hospitals. Within five years, you may receive an exosome therapy yourself. The scientific revolution happening inside your cells right now is about to become the medical revolution that redefines how we treat disease. And it all started with the realization that cells don't just talk—they write letters, pack them in envelopes, and mail them across the body. We're only beginning to read what they say.

Recent breakthroughs in fusion technology—including 351,000-gauss magnetic fields, AI-driven plasma diagnostics, and net energy gain at the National Ignition Facility—are transforming fusion propulsion from science fiction to engineering frontier. Scientists now have a realistic pathway to accelerate spacecraft to 10% of light speed, enabling a 43-year journey to Alpha Centauri. While challenges remain in miniaturization, neutron management, and sustained operation, the physics barriers have ...

Epigenetic clocks measure DNA methylation patterns to calculate biological age, which predicts disease risk up to 30 years before symptoms appear. Landmark studies show that accelerated epigenetic aging forecasts cardiovascular disease, diabetes, and neurodegeneration with remarkable accuracy. Lifestyle interventions—Mediterranean diet, structured exercise, quality sleep, stress management—can measurably reverse biological aging, reducing epigenetic age by 1-2 years within months. Commercial ...

Data centers consumed 415 terawatt-hours of electricity in 2024 and will nearly double that by 2030, driven by AI's insatiable energy appetite. Despite tech giants' renewable pledges, actual emissions are up to 662% higher than reported due to accounting loopholes. A digital pollution tax—similar to Europe's carbon border tariff—could finally force the industry to invest in efficiency technologies like liquid cooling, waste heat recovery, and time-matched renewable power, transforming volunta...

Humans are hardwired to see invisible agents—gods, ghosts, conspiracies—thanks to the Hyperactive Agency Detection Device (HADD), an evolutionary survival mechanism that favored false alarms over fatal misses. This cognitive bias, rooted in brain regions like the temporoparietal junction and medial prefrontal cortex, generates religious beliefs, animistic worldviews, and conspiracy theories across all cultures. Understanding HADD doesn't eliminate belief, but it helps us recognize when our pa...

The bombardier beetle has perfected a chemical defense system that human engineers are still trying to replicate: a two-chamber micro-combustion engine that mixes hydroquinone and hydrogen peroxide to create explosive 100°C sprays at up to 500 pulses per second, aimed with 270-degree precision. This tiny insect's biochemical marvel is inspiring revolutionary technologies in aerospace propulsion, pharmaceutical delivery, and fire suppression. By 2030, beetle-inspired systems could position sat...

The U.S. faces a catastrophic care worker shortage driven by poverty-level wages, overwhelming burnout, and systemic undervaluation. With 99% of nursing homes hiring and 9.7 million openings projected by 2034, the crisis threatens patient safety, family stability, and economic productivity. Evidence-based solutions—wage reforms, streamlined training, technology integration, and policy enforcement—exist and work, but require sustained political will and cultural recognition that caregiving is ...

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.