Ancient Archaea in Your Gut: Rewriting Human Biology

TL;DR: Every day, billions of bacteria from your mouth travel to your gut, where specific pathogens can punch holes in your intestinal barrier and trigger inflammatory diseases affecting your heart, brain, joints, and more. The oral-gut bacterial highway represents a fundamental rethinking of how disease develops and spreads through the body.
Every day, you swallow somewhere between 100 million and 10 billion bacteria from your mouth. Most people brush their teeth thinking about cavities and bad breath, but what's happening in your mouth has consequences far beyond your gums. Scientists have uncovered a biological highway running from your oral cavity straight through to your intestines, and the traffic on this route is reshaping how we understand everything from inflammatory bowel disease to heart attacks.
The oral-gut axis isn't just another trendy microbiome buzzword. It represents a fundamental rethinking of how disease develops and spreads through the human body. When pathogenic bacteria from periodontal disease make the journey south, they don't just pass through harmlessly. They set up shop, disrupt your gut's delicate microbial balance, punch holes in your intestinal barrier, and trigger inflammatory cascades that can reach every organ system.
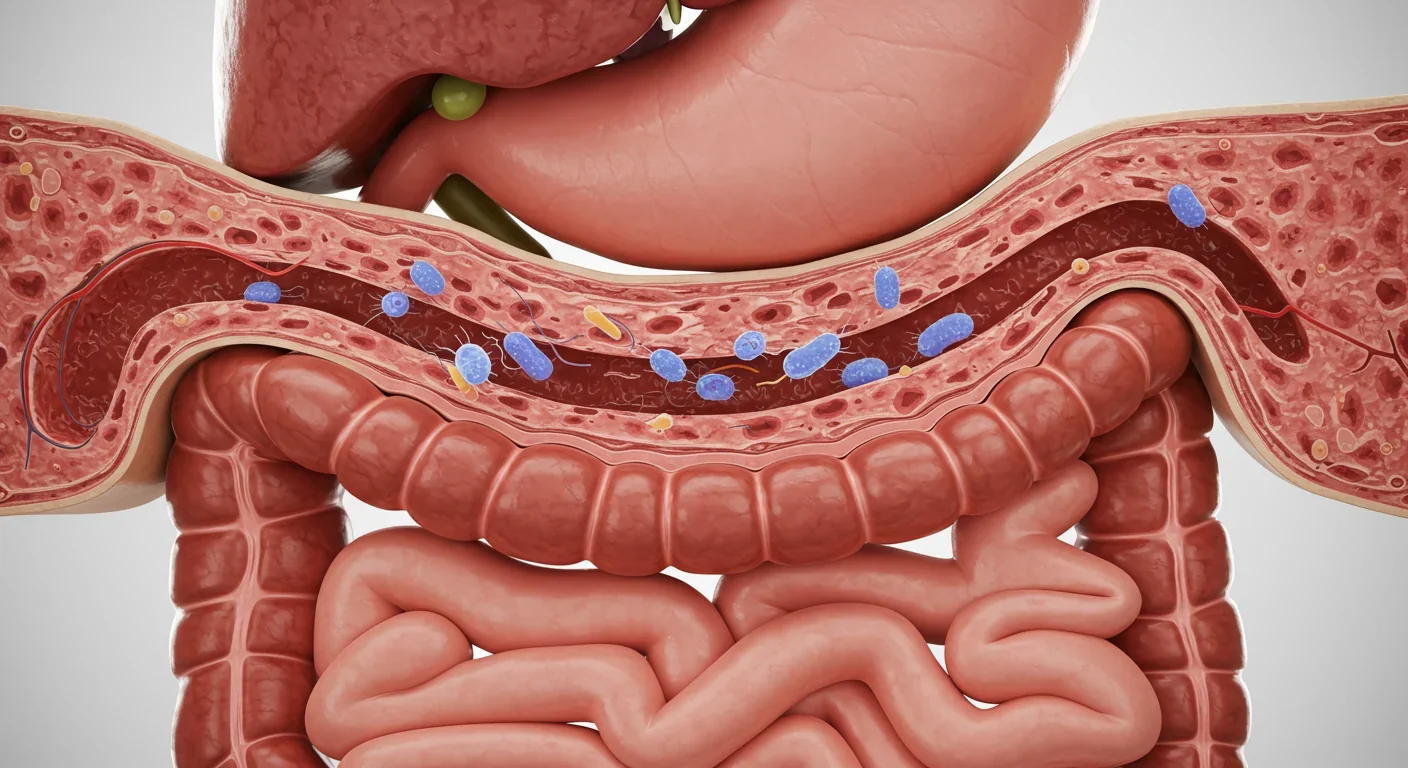
Your mouth hosts one of the most diverse microbial ecosystems in your body, with over 700 species of bacteria calling it home. When gum disease develops, specific pathogenic species like Porphyromonas gingivalis and Fusobacterium nucleatum establish dominance. These aren't passive residents; they're master manipulators of the human immune system.
Every time you swallow, chew, or brush your teeth, you're sending waves of oral bacteria toward your stomach and intestines. Researchers have quantified this daily migration at approximately 100 million to 10 billion P. gingivalis bacteria alone for someone with periodontal disease. That's not a typo. Ten billion bacteria, every single day, making the journey from mouth to gut.
The stomach's harsh acidity used to be considered an effective barrier. But certain oral pathogens have evolved remarkable resilience. P. gingivalis can survive gastric acid exposure and colonize the upper gastrointestinal tract, respiratory tract, and colon. Once these bacteria establish beachheads in your intestines, they begin rewriting the local microbial script.
The translocation doesn't happen through swallowing alone. When gum disease progresses, it creates ulcerations in oral tissue, essentially open doorways to your bloodstream. Bacteria can enter circulation directly through these wounds. Studies have detected oral pathogens in arterial plaque lesions, hundreds of miles from their original habitat. They travel through blood and lymphatic vessels, hitching rides on immune cells that were supposed to destroy them.
Not all bacteria are created equal when it comes to disrupting ecosystems. Porphyromonas gingivalis has earned the designation of "keystone pathogen" because even at low abundance, it can orchestrate widespread dysbiosis. Think of it like an invasive species that doesn't just compete with native organisms but fundamentally alters the environment to favor other problematic species.
The mechanisms behind this are ingeniously destructive. P. gingivalis produces enzymes called gingipains that cleave host antibodies and inflammatory signaling molecules. By degrading IgG1 and IgG3 antibodies, the bacterium effectively blinds part of your immune surveillance system. It also breaks down pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α, creating zones of immune suppression where it and its bacterial allies can thrive.
When these oral invaders reach the gut, they bring this immunological sabotage with them. The intestinal immune system, already balancing the complex task of tolerating beneficial bacteria while attacking pathogens, gets thrown into confusion. The result is inflammation, but not the productive kind that clears infections. Instead, it's chronic, smoldering inflammation that damages tissues while failing to eliminate the bacterial culprits.
Approximately 100 million to 10 billion bacteria from your mouth reach your gut every single day through swallowing alone—and some can survive the journey to establish permanent colonies.
Your intestinal lining is one of the most remarkable barriers in nature: a single-cell-thick wall that must simultaneously absorb nutrients while keeping bacteria and toxins out of your bloodstream. This barrier depends on tight junctions, specialized protein structures that seal the gaps between intestinal cells.
When oral pathogens colonize the gut, one of their primary targets is this barrier. Intestinal permeability increases in response to dysbiosis, creating what researchers call "leaky gut." While that term has been co-opted by wellness marketing, the underlying science is solid and concerning.
The tight junction proteins that normally seal your gut wall—claudins, occludins, and zonulin—can be disrupted by bacterial products and the inflammatory molecules they trigger. Small particles that should never enter your bloodstream, including bacterial fragments, food antigens, and toxins, begin transiting through the paracellular space, the highway between cells.
This breach has systemic consequences. When bacterial components like lipopolysaccharide (LPS) from P. gingivalis enter circulation, they trigger immune responses throughout your body. The liver, heart, brain, joints, and other tissues respond to these signals, initiating inflammatory cascades. What started as gum disease becomes a whole-body problem.
The bacterial migration also disrupts the composition of the gut microbiome itself. Beneficial bacteria that normally dominate the intestinal ecosystem lose ground to opportunistic pathogens. The oral invaders don't need to be the most abundant species to cause havoc; they just need to tip the ecological balance. Research shows that oral pathogen presence correlates with reduced diversity of beneficial gut bacteria and increased populations of pro-inflammatory species.
"Porphyromonas gingivalis is recognized as a 'master of polymicrobial synergy and dysbiosis,' as it engineers its environment and modifies its nutritional demand to survive and persist in the host."
— Frontiers in Cellular and Infection Microbiology
The link between oral health and inflammatory bowel disease has moved from hypothesis to established association. People with periodontal disease show significantly higher rates of Crohn's disease and ulcerative colitis, and the relationship appears to be bidirectional: gut inflammation can worsen oral disease, and oral infection can trigger or exacerbate intestinal inflammation.
Fusobacterium nucleatum, normally a resident of dental plaque, has been detected at elevated levels in the intestinal biopsies of IBD patients. This bacterium doesn't just correlate with disease; experimental studies show it actively promotes intestinal inflammation. When researchers introduced oral bacteria into the guts of animal models, they observed accelerated development of colitis and increased intestinal permeability.

The mechanisms involve multiple pathways. Oral pathogens activate pattern recognition receptors on intestinal cells, triggering inflammatory gene expression. They also interact with the existing gut microbiome, forming polymicrobial communities that are more resistant to immune clearance and more effective at inducing inflammation than either oral or gut bacteria alone.
For patients with IBD, this oral-gut connection has practical implications. Studies evaluating periodontal status in IBD cohorts consistently find worse oral health correlates with more severe intestinal disease. Some researchers now advocate for integrated dental and gastroenterological care for these patients, though this multidisciplinary approach remains rare in clinical practice.
Perhaps the most unsettling discovery about the oral-gut axis involves cancer. Multiple studies have now linked periodontal pathogens to increased risk of colorectal cancer, and the mechanisms are starting to come into focus.
Fusobacterium nucleatum has been detected in colorectal tumor tissue at much higher levels than in healthy adjacent tissue. This bacterium can adhere to cancer cells, promote their proliferation, and suppress anti-tumor immunity. In laboratory studies, when researchers remove F. nucleatum from human colorectal cancer cells, the cancer cells become more susceptible to chemotherapy.
The pathway from oral infection to intestinal cancer likely involves chronic inflammation. Years of low-grade inflammatory signaling in the gut creates an environment where cellular mutations accumulate and escape immune surveillance. Oral pathogens also produce molecules that directly damage DNA. Gingipains from P. gingivalis, for instance, can cleave proteins involved in DNA repair and cell cycle regulation.
Beyond colorectal cancer, oral-gut bacterial translocation has been implicated in esophageal, gastric, and pancreatic cancers. Recent research showed that P. gingivalis can upregulate proliferation and migration of esophageal epithelial cells, leading to chromosomal abnormalities. The oral microbiome's influence extends throughout the digestive tract.
People with severe periodontal disease show up to 50% increased risk for certain cancers, with oral pathogen DNA detected directly in tumor tissue.
The journey of oral bacteria doesn't stop at the gut. Once these pathogens compromise intestinal barrier function and trigger systemic inflammation, cardiovascular consequences follow. Porphyromonas gingivalis has been detected in atherosclerotic plaques, those dangerous fatty deposits that narrow arteries and trigger heart attacks.
Animal studies demonstrate causality. When researchers inoculate mice with P. gingivalis, the bacteria accelerate atherosclerosis development. The bacteria colonize arterial walls, injure the endothelium, and direct local inflammatory responses. They also contribute to blood clot formation, increasing stroke risk.

The mechanisms connecting oral-gut dysbiosis to cardiovascular disease involve multiple pathways. Chronic systemic inflammation from leaky gut elevates inflammatory markers like C-reactive protein and fibrinogen. Bacterial products can directly activate platelets and clotting cascades. The metabolic disruption that follows alters lipid profiles and blood pressure regulation.
Metabolic disease shows similar patterns. Type 2 diabetes and periodontal disease have a bidirectional relationship: diabetes worsens gum disease through immune dysfunction, while periodontal pathogens worsen insulin resistance through systemic inflammation. When oral bacteria colonize the gut and increase intestinal permeability, bacterial endotoxins entering circulation directly interfere with insulin signaling pathways.
The obesity connection adds another layer. Studies show increased intestinal permeability in obese individuals, partly driven by dysbiotic gut microbiomes. Oral pathogen translocation may contribute to this obesity-associated leaky gut, creating a vicious cycle where metabolic disease, oral disease, and gut dysfunction reinforce each other.
One of the most mechanistically interesting aspects of the oral-gut axis involves protein citrullination, a post-translational modification implicated in autoimmune diseases. Porphyromonas gingivalis is unique among bacteria in producing peptidyl arginine deiminase, an enzyme that converts arginine residues in proteins to citrulline.
This matters because rheumatoid arthritis, one of the most common autoimmune diseases, is characterized by antibodies against citrullinated proteins. The hypothesis gaining traction is that periodontal infection may initiate the autoimmune cascade. Gingipain enzymes cleave host proteins to expose arginine residues, then bacterial peptidyl arginine deiminase citrullinates these proteins. The modified proteins get deposited in tissues throughout the body, including joints, where the immune system recognizes them as foreign.
Clinical data supports this connection. Patients with rheumatoid arthritis have higher rates of periodontal disease, and periodontal treatment can reduce rheumatoid arthritis disease activity in some patients. The oral-gut translocation of P. gingivalis may seed autoimmune responses not just locally but systemically, as citrullinated proteins circulate and deposit in distant tissues.
Other autoimmune conditions show similar patterns. Multiple sclerosis, systemic lupus erythematosus, and inflammatory arthritis have all been linked to increased intestinal permeability and dysbiosis. While oral bacteria aren't the sole cause, they may act as environmental triggers in genetically susceptible individuals.
"The gingipains of P. gingivalis cleave host IgG1/IgG3 antibodies and pro-inflammatory cytokines, impairing the host immune response and allowing bacterial persistence."
— Wikipedia: Porphyromonas gingivalis
Recent discoveries have extended the reach of oral pathogens all the way to the brain. Porphyromonas gingivalis DNA and its toxic proteases have been detected in the brains of Alzheimer's disease patients, raising provocative questions about whether periodontal disease might contribute to neurodegenerative disease.
The proposed pathway involves several steps. Oral bacteria can reach the brain through multiple routes: systemic circulation after entering through diseased gums, nerve pathways including the trigeminal and olfactory nerves, or through a compromised blood-brain barrier made leaky by chronic systemic inflammation originating in the gut.

Once in neural tissue, bacterial products like gingipains can trigger neuroinflammation and may contribute to the accumulation of amyloid-beta, the protein that forms plaques in Alzheimer's disease. Some researchers propose that amyloid-beta might actually be part of the brain's antimicrobial defense system, and its accumulation could represent a response to chronic bacterial presence.
This remains one of the most controversial aspects of oral-gut-brain research. Skeptics point out that detecting bacterial DNA in diseased tissue doesn't prove causation; the bacteria might colonize after disease develops rather than causing it. Nevertheless, pharmaceutical companies have launched clinical trials testing gingipain inhibitors as potential Alzheimer's treatments, suggesting the hypothesis is being taken seriously.
Understanding the oral-gut axis opens new intervention possibilities beyond traditional dental care and gastroenterology. The most obvious starting point is aggressive periodontal treatment for patients with systemic diseases. Several studies have shown that treating periodontal disease can improve markers of systemic inflammation and, in some cases, disease activity in conditions like diabetes and rheumatoid arthritis.
Probiotics represent another frontier. Certain strains like Lactobacillus rhamnosus, L. reuteri, and Faecalibacterium prausnitzii have been shown to reduce intestinal permeability in both animal models and human trials. The question is whether probiotics specifically selected to counteract oral pathogen effects might offer benefits beyond general gut health support.
Prebiotics—dietary fibers that nourish beneficial bacteria—also show promise. By supporting the growth of beneficial gut bacteria, prebiotics might help these microbes compete against oral pathogen colonizers. Some researchers advocate for combined prebiotic and probiotic approaches to rebuild healthy gut ecosystems while simultaneously reducing pathogen load.
Drug development targeting specific bacterial virulence factors is advancing. Gingipain inhibitors are the most advanced, with compounds showing ability to reduce P. gingivalis pathogenicity without killing the bacteria. This approach might avoid some of the dysbiosis problems caused by broad-spectrum antibiotics.
Vaccines represent the holy grail of prevention. Experimental vaccines using capsular polysaccharide from P. gingivalis have reduced oral bone loss in mouse models, inducing strong antibody responses. A human periodontal disease vaccine could theoretically prevent not just gum disease but its systemic consequences.
Barrier restoration therapies are also in development. Larazotide acetate, a zonulin receptor antagonist that has been tested for celiac disease, aims to strengthen tight junctions and reduce intestinal permeability. Similar approaches might help patients whose gut barriers have been compromised by oral pathogen translocation.
Treating periodontal disease has been shown to reduce inflammatory markers and improve outcomes in diabetes, rheumatoid arthritis, and cardiovascular disease—suggesting oral health interventions may have body-wide benefits.
Despite rapid progress, major questions remain unanswered. The relative contribution of different translocation pathways—swallowing, bloodstream invasion, lymphatic spread—hasn't been definitively quantified in humans. Animal models provide clues, but the dynamics in human bodies with complex chronic diseases may differ substantially.
Individual variation poses another puzzle. Not everyone with periodontal disease develops IBD, cardiovascular disease, or other systemic conditions. Genetic susceptibility, existing gut microbiome composition, diet, stress, and other environmental factors all modulate outcomes. Identifying who faces highest risk from oral-gut bacterial translocation would enable targeted interventions.
The temporal sequence needs clarification. Does oral pathogen gut colonization precede disease onset, or do systemic conditions create opportunities for oral bacteria to establish intestinal footholds? Longitudinal studies tracking people from health through disease development are expensive and time-consuming but essential for answering causation questions.
Intervention trial results will be crucial. Can intensive periodontal treatment prevent disease progression in high-risk individuals? Do probiotics selected to compete with oral pathogens provide benefits beyond standard strains? Will vaccines prove effective and safe? These questions require rigorous randomized controlled trials, not just observational associations.
For all the excitement about oral-gut axis research, translation to clinical practice has been slow. Dentists and gastroenterologists rarely collaborate, and patients' oral and intestinal health are typically managed in complete isolation. Insurance systems reinforce this fragmentation, rarely covering dental care as medical treatment even when oral disease clearly contributes to systemic illness.
Some medical centers have started integrated oral-systemic health clinics where dentists, gastroenterologists, rheumatologists, and other specialists coordinate care. These remain rare exceptions rather than standard practice. Most patients with both periodontal disease and systemic illness never receive information about the connections between their conditions.
The evidence base, while growing, still has gaps that make clinical recommendations challenging. Should every patient with IBD receive intensive periodontal screening and treatment? Should everyone with severe gum disease undergo gut microbiome testing? The studies haven't yet defined thresholds and protocols that justify such sweeping practice changes.
The oral-gut axis research forces a fundamental reconceptualization of disease. The outdated model of discrete, organ-specific pathologies doesn't fit the microbial reality of human bodies. Your mouth, gut, bloodstream, and distant organs form an interconnected ecosystem where bacterial populations in one location can determine disease risk in anatomically distant sites.
This ecological view of health emphasizes context over simple presence or absence. The same bacterial species might be harmless in one environment but pathogenic in another. P. gingivalis in low numbers in a healthy oral microbiome may cause no problems, but when it blooms during periodontal disease and translocates to the gut, it becomes a systemic threat.
The implications extend beyond treating existing disease to preventing it. Maintaining oral health isn't just about saving teeth; it's about protecting your entire body from microbial invasions that can seed chronic diseases decades later. The daily hygiene ritual of brushing and flossing, often dismissed as cosmetic, takes on new significance as frontline immune defense.
Public health messaging has barely begun to incorporate these insights. People understand that smoking causes cancer and that exercise protects the heart, but the connection between oral hygiene and systemic disease remains obscure to most. As evidence strengthens, we may see campaigns positioning dental care as essential preventive medicine rather than optional maintenance.
The next decade of oral-gut axis research will likely deliver surprises. As sequencing technology improves and becomes cheaper, we'll track individual bacterial strains' movements through the body with unprecedented precision. Longitudinal microbiome studies will reveal how oral and gut communities co-evolve over lifetimes and how interventions ripple through these ecosystems.
Artificial intelligence and machine learning will help parse the staggering complexity of multi-omics data—combining genomics, transcriptomics, proteomics, and metabolomics—to identify patterns invisible to human analysis. Predictive models might eventually forecast systemic disease risk from oral microbiome profiles, enabling preventive interventions years before symptoms appear.
Personalized microbiome therapeutics represent the frontier. Rather than generic probiotics, we might use detailed analysis of individuals' oral and gut microbiomes to design customized bacterial cocktails or phage therapies that target specific pathogens while preserving beneficial species. CRISPR and other gene-editing tools could potentially modify pathogenic bacteria to render them harmless without eliminating them entirely.
The regulatory landscape will need to evolve. Current frameworks struggle with microbiome-based therapies that blur boundaries between drugs, foods, and biologics. As interventions targeting the oral-gut axis advance from laboratory to clinic, regulatory agencies will need to develop appropriate oversight that ensures safety without stifling innovation.
Your mouth is not isolated from the rest of your body. The bacteria residing in your oral cavity can, and do, travel to your intestines and beyond, carrying the potential to trigger or worsen systemic diseases. Periodontal disease isn't just about tooth loss; it's a risk factor for inflammatory bowel disease, cardiovascular disease, diabetes, certain cancers, and possibly even neurodegenerative conditions.
This isn't cause for panic but for perspective. Oral health deserves the same attention as diet, exercise, and other lifestyle factors that influence long-term health outcomes. Regular dental care, proper hygiene, and early treatment of gum disease aren't vanity projects; they're investments in whole-body health.
The oral-gut axis reminds us that human biology is fundamentally ecological. We're not just human cells but complex ecosystems where microbial inhabitants outnumber our own cells. Understanding and managing these microbial communities—in our mouths, guts, and throughout our bodies—will increasingly define 21st-century medicine. The highway running from oral cavity to intestines carries traffic in both directions, and managing this route may hold keys to preventing and treating some of medicine's most challenging chronic diseases.
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.