Ancient Archaea in Your Gut: Rewriting Human Biology

TL;DR: Your gut contains 10^15 viruses that wage constant warfare on bacteria, shaping your microbiome and health. New research reveals these bacteriophages are precision tools for treating antibiotic-resistant infections and chronic diseases.
Inside your intestines right now, a war is raging. Not the immune battles you've heard about, but something stranger and more fundamental: trillions of viruses are hunting, infecting, and killing bacteria in a continuous microscopic arms race. These aren't the viruses that make you sick. They're bacteriophages, the most abundant biological entities on Earth, and they're rewriting what we know about gut health.
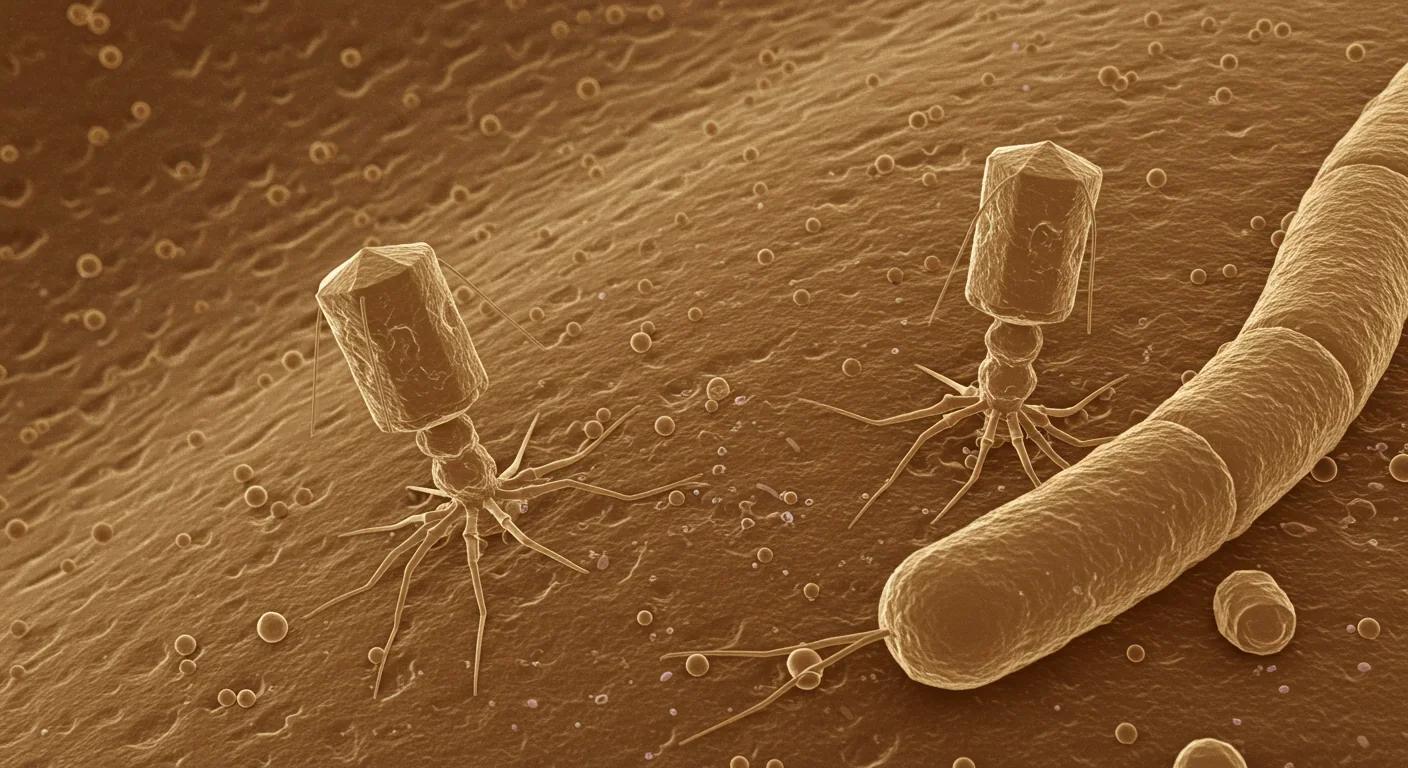
For decades, we've focused on bacteria as the stars of the microbiome story. Probiotics, prebiotics, fermented foods—all designed to nurture the "good bacteria" while fighting the bad. But researchers have discovered something remarkable: your gut contains roughly 10^15 viral particles, with approximately 90% being bacteriophages that exist solely to infect bacteria. These viruses aren't passive hitchhikers. They're active architects of your microbiome, precise biological weapons that can eliminate harmful bacteria, promote beneficial species, and maintain the delicate ecological balance your health depends on.
This revelation is transforming medicine. Scientists are now developing phage therapy treatments for antibiotic-resistant infections, manipulating viral populations to treat inflammatory bowel disease, and discovering that the invisible viral ecosystem in your gut may influence everything from obesity to immune function. The bacteria-virus warfare happening inside you isn't just fascinating biology—it might be the key to solving some of medicine's most stubborn problems.
When scientists first sequenced the human microbiome in the early 2000s, they found something puzzling. Genetic material everywhere that didn't match any known bacteria. Much of it belonged to viruses, but the technology and methods to study these microscopic predators didn't exist yet. So the virome—the collective community of viruses living in and on us—remained largely ignored.
That changed around 2010 when metagenomics technology advanced enough to identify viral particles in fecal samples. Using epifluorescence microscopy, researchers counted viral loads reaching 10^12 virus-like particles per gram of stool. The numbers were staggering. For every bacterial cell in your gut, there are roughly ten viral particles. Most belong to a class called Caudovirales, tailed bacteriophages that look like lunar landers under electron microscopes.
Your gut contains 10^15 viral particles—that's 10 viruses for every bacterial cell. Most are bacteriophages that exist solely to hunt and kill bacteria, making them the most abundant predators in your body.
The gut virome comprises three main groups: bacteriophages that infect prokaryotic cells, eukaryotic viruses that target human and animal cells, and human endogenous retroviruses integrated into our DNA from ancient infections. But bacteriophages dominate—both in numbers and in their impact on gut ecology. Unlike broad-spectrum antibiotics that wipe out beneficial and harmful bacteria alike, each phage species typically targets just one or a few bacterial strains with surgical precision.
This specificity makes phages nature's smart weapons. A Chinese gut virus catalogue published in 2025 identified thousands of distinct viral species in the human gut, each with specific bacterial prey. The catalogue revealed that virome composition varies dramatically between individuals and changes based on diet, geography, age, and health status. Your viral fingerprint is as unique as your bacterial microbiome—and possibly more important.

Bacteriophages kill bacteria in two ways, and the balance between these strategies determines the structure of your gut ecosystem. In lytic infection, the phage injects its DNA into a bacterial cell, hijacks the cellular machinery to make copies of itself, then bursts the cell open to release dozens of new phages. It's brutal, efficient, and keeps bacterial populations in check. This predator-prey dynamic prevents any single bacterial species from dominating the gut ecosystem.
The second strategy is more subtle. In lysogenic infection, phage DNA integrates into the bacterial chromosome as a dormant prophage. The virus hitchhikes, replicating along with bacterial DNA for generations. But prophages aren't just freeloaders. They often carry genes that alter bacterial metabolism, antibiotic resistance, or virulence. A harmless gut bacterium can become pathogenic when a prophage activates, encoding toxins or factors that help the host bacterium colonize new niches.
Research shows that the balance between these lifecycles matters tremendously for health. In inflammatory bowel disease patients, the phageome shifts from primarily lytic to predominantly lysogenic. This means more prophages integrating into bacterial genomes, potentially transferring genes that make bacteria more aggressive or resistant to immune responses. The data is striking: Caudovirales levels rise while their diversity declines in IBD, and Microviridae abundance decreases in Crohn's disease but increases in ulcerative colitis.
"The phageome in IBD patients shifted from a virulent (lytic) to a temperate (lysogenic) life cycle, resulting in bacteriophage genes coexisting in equilibrium with the host."
— BMC Medicine research review on gut virome in inflammatory bowel disease
This viral population shift isn't just a symptom of disease—it may be a cause. When phages switch to lysogenic strategies, they stop killing bacteria as aggressively. Bacterial diversity drops because the viral predators that normally prevent overgrowth of dominant species are essentially turned off. The result is dysbiosis: an unbalanced microbial community where harmful bacteria proliferate unchecked.
The Chinese gut virus catalogue study mapped these disease-related viral signatures across thousands of individuals. They found specific phage species associated with type 2 diabetes, colorectal cancer, and cirrhosis. Some viruses appear protective, others pathogenic. The composition of your virome—not just your bacterial microbiome—predicts disease risk.

Your gut isn't just a tube full of bacteria and viruses. The intestinal lining is coated with a thick mucus layer made primarily of MUC2 protein, creating a physical and chemical barrier between the microbial world and your body. Bacteriophages interact with this barrier in surprising ways that influence immune function and intestinal permeability.
Some phages carry a protein called Hoc on their capsid surface. Hoc binds directly to MUC2 proteins, embedding the phage in the mucus layer. This interaction triggers increased mucus production, thickening the barrier and preventing bacterial invasion. Essentially, phages recruit your own cells to strengthen defenses. Studies show that phage-encoded Hoc proteins can reduce inflammatory responses by physically separating bacteria from intestinal epithelial cells.
But other phages do the opposite. They encode enzymes called depolymerases that break down extracellular matrix components, including mucus. These phages use their enzymatic arsenal to penetrate biofilms where their bacterial targets hide. The downside? They can also degrade the protective mucus layer, increasing intestinal permeability and allowing bacteria and bacterial products to contact immune cells, triggering inflammation.
This dual role of phages—both strengthening and weakening the mucus barrier—suggests that phage species composition, not just abundance, determines their net effect on gut homeostasis. An imbalanced virome with too many depolymerase-producing phages might contribute to leaky gut syndrome and chronic inflammation. The immune system doesn't just fight bacteria; it responds to the viral ecosystem that controls bacterial access to your cells.
Bacteriophages can both strengthen and weaken your intestinal barrier. Some phage proteins bind to mucus and trigger increased production, while others break down the protective layer. The balance between these phage types may determine susceptibility to inflammatory gut diseases.
Research has found that individuals with inflammatory bowel disease show altered phage-mucus interactions. The protective phages that normally reinforce the barrier decrease, while phages associated with mucus degradation increase. This creates a vicious cycle: barrier breakdown allows bacterial invasion, triggering inflammation that further disrupts the microbiome and virome. Breaking this cycle requires understanding and potentially manipulating the viral players.
When you take antibiotics, you know they kill bacteria. What you probably don't know is that they also trigger a massive reorganization of your gut virome—and those viral changes can persist long after the drugs are gone. A 2025 study on stool donors tracked the gut microbiome and virome before and after antibiotic treatment, revealing that bacterial communities don't simply bounce back.
Antibiotics caused lasting disruption to bacterial communities, significantly reducing important taxa like Bifidobacterium bifidum, Blautia wexlerae, and Akkermansia muciniphila. These aren't random casualties—they're keystone species that produce short-chain fatty acids, maintain the mucus layer, and regulate immune function. Their loss creates ecological vacancies in the gut.
Here's where phages get interesting. The study found that virome composition shifted post-antibiotic, with a decrease in viral diversity but an increase in crAssphage abundance. CrAssphage is one of the most common bacteriophages in the human gut, but its exact role remains mysterious. The surge in crAssphage after antibiotics suggests it may help recolonize the gut by targeting bacterial populations that expand during the post-antibiotic chaos.
This virome reshaping has profound implications for understanding why antibiotics cause long-term health problems. It's not just bacterial depletion. The viral predators that normally maintain microbial balance are thrown into disarray. Some phages lose their bacterial hosts entirely and disappear. Others proliferate wildly as opportunistic bacteria expand. The result is a fundamentally different ecosystem that may take months or years to restabilize—if it ever fully does.
The study recommended that stool donors be quarantined for at least three months after antibiotic exposure to prevent transmission of altered viromes. This precaution highlights an uncomfortable truth: we've been using antibiotics for nearly a century without understanding their impact on the viral ecosystem that governs bacterial communities. Every course of antibiotics is an ecological intervention with ripple effects we're only beginning to trace.
For all the problems antibiotics cause, they at least kill bacteria. But antibiotic resistance is erasing that advantage. By 2050, resistant infections could kill 10 million people annually if current trends continue. Bacteriophages offer an alternative that's been hiding in plain sight for a century.
Phage therapy—using viruses to kill pathogenic bacteria—was discovered in 1917 by French-Canadian microbiologist Félix d'Hérelle. He isolated bacteriophages from the stool of recovering dysentery patients and used them to treat bacterial infections successfully. But antibiotics arrived in the 1940s, proving cheaper and easier to produce. Western medicine largely abandoned phage research, though scientists in the Soviet Union, Georgia, and Poland continued developing phage treatments.
Now phage therapy is experiencing a renaissance. A comprehensive 2024 review examined its safety and efficacy across numerous conditions. For gastrointestinal infections—including Clostridioides difficile, pathogenic E. coli, Salmonella, and Shigella—phage cocktails demonstrated remarkable effectiveness. They directly lysed pathogenic bacteria while sparing beneficial commensals, the precise targeting antibiotics can't achieve.
"Phage therapy has been demonstrated to be safe and effective in treating a range of gastrointestinal infections by directly lysing pathogenic bacteria while sparing commensals."
— MDPI comprehensive review on phage therapy safety and efficacy
Clinical trials are yielding promising data. One study administered oral phage cocktails to healthy adults for 28 days, finding them well-tolerated with reduced inflammatory markers. Another trial in piglets with E. coli diarrhea showed that phage treatment improved outcomes without disrupting overall microbiota composition. For C. difficile infection in hamsters, a phage cocktail delayed symptom onset and reduced mortality.
The most exciting development involves engineered phages. Researchers have created CRISPR-Cas3-enhanced bacteriophages (LBP-EC01) designed to cleave antibiotic resistance genes directly in target bacteria. Early human trials for urinary tract infections showed these precision-engineered viruses were safe and effective. This represents a leap beyond natural phages: viruses designed not just to kill bacteria but to dismantle the genetic machinery of antibiotic resistance itself.

Phage therapy can be delivered orally, enterally, or topically with high tolerability across patient populations—infants, adults, immunocompromised individuals. The viruses replicate at the site of infection, amplifying their own therapeutic effect, then decline once their bacterial targets are eliminated. Unlike antibiotics that persist in the environment promoting resistance, phages are self-limiting biological agents.
If the virome shapes bacterial communities, and bacterial communities influence health, then manipulating the virome becomes a therapeutic strategy. This goes beyond phage therapy for acute infections. Researchers are exploring how to engineer viral ecosystems to treat chronic conditions.
Inflammatory bowel disease is the obvious target. Since IBD involves characteristic virome alterations—elevated Caudovirales, reduced Microviridae diversity, increased lysogenic phage integration—correcting these imbalances could reduce inflammation. Clinical approaches include phage cocktails that restore viral diversity, engineered phages targeting pathobionts that drive inflammation, and fecal virome transplants that transfer healthy viral communities.
The concept of virome transplantation parallels fecal microbiota transplantation but focuses specifically on transferring viral particles. A 2025 study found that engrafted bacteriophages from donor stool could modulate bacterial metabolism in recipients, suggesting the virome is a transferable therapeutic agent. The viruses established stable populations and continued influencing bacterial community structure months after transplantation.
Obesity and metabolic disorders are also potential targets. The Chinese gut virus catalogue identified specific viral signatures associated with type 2 diabetes. If those viruses contribute to disease by promoting certain bacterial populations or metabolic pathways, phage interventions might help. Animal studies are exploring this possibility, though human trials remain years away.
The advantage of virome manipulation over direct bacterial interventions is specificity. Probiotics deliver live bacteria, but they rarely colonize permanently and can be overwhelmed by existing communities. Phages, by contrast, actively hunt and eliminate target bacteria while promoting others. They're ecosystem engineers rather than passive supplements. A carefully designed phage cocktail could systematically reshape bacterial populations toward a healthier equilibrium.
The future of gut health may lie not in probiotics, but in precisely engineered bacteriophage cocktails that reshape bacterial populations by eliminating harmful species and promoting beneficial ones—ecosystem engineering at the microscopic scale.
But there are challenges. Phages can transfer genes between bacteria through transduction, potentially spreading antibiotic resistance genes or virulence factors. Lysogenic phages integrate into bacterial genomes unpredictably. Some phages provoke immune responses. The long-term stability of introduced viral populations remains uncertain. And we still don't understand enough about healthy virome composition to know what we're aiming for.
Your virome isn't fixed. Like your bacterial microbiome, it responds to environmental factors—diet, stress, sleep, medications. Understanding these influences opens possibilities for supporting a healthy viral ecosystem without direct medical intervention.
Diet appears particularly important, though research is just beginning. Fermented foods—yogurt, kefir, sauerkraut, kimchi—deliver phages along with bacteria. These viruses come from the bacterial cultures used in fermentation and can temporarily colonize the gut. Whether they provide lasting benefits remains unclear, but they expose your microbiome to diverse viral species that may enhance resilience.
Fiber intake influences the virome indirectly by promoting beneficial bacteria. A diverse bacterial community creates more ecological niches for different phage species, increasing viral diversity. High-fiber diets that support bacterial diversity likely support viral diversity too. Conversely, highly processed diets that reduce bacterial diversity may impoverish the virome, making the microbial ecosystem more vulnerable to disruption.
Antibiotics are the most disruptive factor. As we've seen, they trigger lasting virome changes that persist long after bacterial populations rebound. This doesn't mean avoiding antibiotics when medically necessary, but it suggests we should use them more judiciously and explore ways to support virome recovery afterward—perhaps through dietary interventions or even therapeutic phage supplementation.
Stress, inflammation, and immune function all influence gut ecology. Chronic stress alters gut motility and secretions, changing the environment where microbes and viruses live. Immune dysfunction can disrupt the careful balance between tolerance of beneficial microbes and defense against pathogens. Since phages interact with immune cells and the mucus barrier, these factors likely affect viral communities, though specific mechanisms remain poorly understood.
Age is another factor. Infant viromes are unstable and diverse as the microbiome establishes itself. Adult viromes tend to be more stable but less diverse. Elderly individuals show further virome changes that correlate with bacterial dysbiosis and frailty. Whether these age-related viral shifts contribute to decline or merely reflect other aging processes is an open question with profound implications for longevity research.
The bacteria-virus warfare in your gut isn't a one-sided slaughter. Bacteria have evolved sophisticated defenses against phage infection, and phages have evolved countermeasures. This evolutionary arms race plays out at speeds that dwarf human evolution, with bacterial generation times measured in minutes and viral replication measured in hours.
Bacteria employ multiple defense systems. The most famous is CRISPR-Cas, a molecular memory bank that stores DNA sequences from previous phage infections. When a phage attacks again, the CRISPR system recognizes its DNA and destroys it. Bacteria also restrict phage DNA entry, abort their own reproduction to prevent phage amplification, and deploy anti-phage toxins. Some bacteria form biofilms—cooperative communities encased in protective matrices that phages struggle to penetrate.
Phages counter with equal creativity. They mutate rapidly, evading CRISPR recognition. They encode anti-CRISPR proteins that disable bacterial defenses. Some phages communicate with each other through small molecules, coordinating infection strategies. Others carry genes that protect their bacterial hosts from competing phages, turning bacteria into viral fortresses.
This arms race drives innovation on both sides. Bacteria acquire new defense systems through horizontal gene transfer—swapping genes with other bacteria or incorporating them from phages. Phages accumulate genetic diversity through mutation and recombination. The result is a coevolutionary dynamic where neither side wins permanently. Your gut contains thousands of these microscopic conflicts simultaneously, each influencing the others through a web of ecological interactions.
Importantly, this warfare isn't necessarily bad for you. The constant pressure phages exert on bacteria prevents any single species from dominating. It maintains diversity. It forces bacteria to invest resources in defense rather than virulence. Some researchers argue that the phage-bacteria arms race is essential for ecosystem stability, creating dynamic equilibrium through perpetual conflict.
The flip side is that disrupting this balance—through antibiotics, disease, or environmental change—can trigger cascading failures. If beneficial bacteria lose their phage predators, they might not thrive as expected; they might be outcompeted by opportunistic species. If pathogenic bacteria escape viral control, they can cause disease before the immune system responds. The arms race is part of the ecosystem's regulatory machinery, and like any complex system, it's more fragile than it appears.
We're at the beginning of understanding the virome's role in health and disease. The technology to sequence viral genomes at scale only emerged in the last decade. Most published research focuses on bacterial microbiomes. The Prophage-DB database launched in 2024, cataloging prophages across environmental samples, illustrates how basic our knowledge remains. We're still mapping the territory.
But the pace is accelerating. Large-scale projects like the Chinese gut virus catalogue are characterizing viral diversity across populations. AI and machine learning tools are predicting phage-bacteria interactions from genomic data. Engineered phage platforms incorporating CRISPR and synthetic biology are entering clinical trials. Within five to ten years, personalized virome analysis could become part of routine medical care.
The therapeutic possibilities are extraordinary. Imagine a world where antibiotic-resistant infections are treated with designer phage cocktails tailored to the specific bacteria in your body. Where chronic inflammatory conditions respond to virome rebalancing rather than lifelong immune suppression. Where we manipulate viral ecosystems to prevent obesity, improve mental health, or enhance longevity. These aren't science fiction—they're logical extensions of current research trajectories.
But there are also profound unknowns. We don't know the long-term consequences of introducing synthetic phages into natural ecosystems. We don't understand how virome manipulation might affect evolution, both of bacteria and viruses. Phage therapy faces regulatory hurdles because viruses are living, evolving entities that don't fit neatly into pharmaceutical frameworks designed for chemical drugs. And there's always the risk of unintended consequences when we intervene in complex ecological networks we barely comprehend.
The deeper challenge is conceptual. For a century, we've thought about fighting infections—killing germs, eliminating pathogens, sterilizing spaces. The virome suggests a different approach: managing ecosystems, balancing populations, guiding evolution. Medicine becomes less about warfare against microbes and more about ecological stewardship of the invisible civilizations within us.
The bacteriophage warfare in your gut will never end. It's been happening for hundreds of millions of years, since bacteria and viruses first evolved. It will continue as long as microbes exist. This isn't a problem to solve but a process to understand and, perhaps, gently guide.
Your health depends on this viral army you never knew existed. The trillions of phages inside you are hunting, infecting, killing bacteria every moment. They're shaping communities, transferring genes, interacting with your immune system, and maintaining the ecological balance that separates health from disease. You are not just a human with a bacterial microbiome. You're a complex ecosystem where viruses govern bacterial populations that influence your metabolism, immunity, and possibly even your brain.
"The gut virome is a community including viruses that infect eukaryotic and prokaryotic cells, with approximately 90% of the gut's 10^15 viruses being part of the phageome."
— BMC Medicine research review
This perspective is humbling. We are not autonomous individuals but walking ecosystems. Our bodies are habitats for vast communities of microbes locked in perpetual competition and cooperation. The boundaries between self and other blur when you realize that microbial genes outnumber human genes a hundredfold in your body, and viral genes outnumber bacterial genes several-fold.
But this perspective is also empowering. If health emerges from ecological balance, we can support that balance through diet, lifestyle, and thoughtful medical interventions. If disease reflects ecological collapse, we can develop therapies that restore equilibrium rather than blasting ecosystems with antibiotics. The virome revolution offers a path toward more sophisticated, sustainable medicine.
The hidden viral army inside your gut has been waging war on your behalf since birth. Now, finally, we're learning to work with them instead of ignoring their existence. What we do with that knowledge may determine whether the next century sees humanity succumb to resistant infections or finds new ways to thrive alongside the microbial and viral worlds we've always carried within us. The war goes on. We're just beginning to understand whose side we should be on.
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.