Ancient Archaea in Your Gut: Rewriting Human Biology

TL;DR: Pharmacogenomic testing uses genetic information to predict how patients will respond to medications, potentially preventing thousands of adverse drug reactions annually. Major healthcare systems are implementing preemptive testing programs, though insurance coverage and equity challenges remain.
Sarah Martinez's doctor prescribed clopidogrel after her heart attack, but a simple genetic test revealed something crucial: her body couldn't metabolize the drug effectively. Without that test, she might have walked out of the hospital with a prescription that wouldn't protect her. Instead, her physician chose an alternative that her genetic profile showed would work. This scenario is becoming more common across healthcare systems, as pharmacogenomics transforms how we think about prescribing medications.
Adverse drug reactions now rank as the third leading cause of death in the United States, claiming over 128,000 lives annually. But here's what makes this tragedy particularly frustrating: genetic factors account for over 60% of these reactions, and we now have the tools to identify many of them before anyone takes a single pill.
Pharmacogenomics sounds technical, but the concept is straightforward. Your genetic code contains instructions for producing enzymes that break down medications. Some people metabolize drugs quickly, others slowly, and these differences can mean the gap between a life-saving treatment and a medical emergency.
The science behind this isn't new. Researchers have understood for decades that genetic variations affect drug metabolism, but the technology to make testing practical and affordable has only recently arrived. What once required specialized laboratory equipment and weeks of analysis can now happen with a cheek swab and a few days of processing.
Pharmacogenomics accounts for approximately 80% of variability in how drugs work in different people's bodies - not a small effect buried in statistical noise, but the difference between a medication that helps and one that harms.
The numbers tell a compelling story. Pharmacogenomics accounts for approximately 80% of variability in how drugs work in different people's bodies. That's not a small effect buried in statistical noise - it's the difference between a medication that helps and one that harms.
Consider the CYP2D6 gene, which produces an enzyme responsible for metabolizing about 25% of prescription medications. Between 5-10% of people carry genetic variants that make them poor metabolizers, meaning standard doses can build up to toxic levels in their systems. Another 1-2% are ultra-rapid metabolizers who break down drugs so quickly they get little benefit from normal dosing.
The shift from research curiosity to clinical reality has accelerated dramatically. Major academic medical centers now implement preemptive pharmacogenomic testing, running panels of genetic markers before patients even need the medications they test for. The logic makes sense: test once, use the information for a lifetime of prescribing decisions.
Vanderbilt University Medical Center pioneered this approach with their Pharmacogenomic Resource for Enhanced Decisions in Care and Treatment (PREDICT) program. They've tested over 30,000 patients, integrating results directly into electronic health records. When a physician prescribes a medication affected by a patient's genetic profile, alerts pop up automatically, suggesting dose adjustments or alternative medications.
Clinical implementation studies show impressive results. At institutions using pharmacogenomic testing for cardiovascular medications, researchers documented a 30% reduction in adverse drug events. For psychiatric medications, where trial-and-error prescribing has traditionally been the norm, genetic testing helped patients find effective treatments an average of six weeks faster.
The Clinical Pharmacogenetics Implementation Consortium has published guidelines for over 80 gene-drug pairs, giving physicians evidence-based recommendations for adjusting prescriptions based on genetic results. These aren't experimental protocols - they represent the current standard of care for institutions serious about precision medicine.
Not all medications benefit equally from pharmacogenomic testing. Some categories show particularly strong evidence, making them prime candidates for genetic screening.
Cardiovascular medications top the list. Clopidogrel, a blood thinner prescribed to millions after heart attacks or strokes, requires activation by the CYP2C19 enzyme. People with reduced-function variants of this gene get significantly less protection from blood clots. The FDA added a warning to clopidogrel's label back in 2010, but many patients still don't receive testing before their prescriptions.
"Genetic-guided dosing reduces hospitalizations by 30% and cuts healthcare costs significantly."
— Clinical research on warfarin pharmacogenomics
Warfarin dosing provides another compelling example. This widely-used anticoagulant has a notoriously narrow therapeutic window - too little and blood clots form, too much and dangerous bleeding occurs. Genetic variants in CYP2C9 and VKORC1 can predict optimal dosing far more accurately than the traditional trial-and-error approach. Studies show genetic-guided dosing reduces hospitalizations by 30% and cuts healthcare costs significantly.
Psychiatric medications present a different challenge. Depression, anxiety, and other mental health conditions often require trying multiple medications before finding one that works. Pharmacogenetic testing for psychiatric drugs can shorten this frustrating process. Companies like GeneSight and Genomind have built their businesses around panels specifically designed for mental health prescribing.

Cancer treatments increasingly rely on pharmacogenomic information. Many chemotherapy drugs work by exploiting specific genetic vulnerabilities in tumor cells, but they can also reveal problematic variants in patients' own genes. Testing for TPMT variants before prescribing thiopurines, for instance, prevents life-threatening bone marrow suppression in the 0.3% of people who lack functional copies of this gene.
Pain medications carry particular importance given the opioid crisis. Some people metabolize codeine into morphine extremely rapidly due to CYP2D6 variants, creating overdose risk at standard doses. Others get no pain relief because they can't make the conversion at all. Genetic testing before prescribing opioids could prevent both inadequate pain control and accidental overdoses.
Pharmacogenomic testing isn't free, though costs have plummeted. Basic panels testing a handful of key genes run $200-500. Comprehensive panels examining dozens of genes can reach $2,000-3,000, though prices continue dropping as the technology matures.
The economics look increasingly favorable. Studies on cardiovascular pharmacogenomics show that preemptive testing saves the healthcare system money within the first year by preventing adverse drug reactions requiring emergency department visits, hospitalizations, or intensive care.
One analysis calculated that testing Medicare patients before prescribing cardiovascular medications would save $3.7 billion over five years. The math works because preventing a single serious adverse drug reaction - which can cost tens of thousands of dollars - pays for testing many patients.
Preventing a single serious adverse drug reaction can cost tens of thousands of dollars - enough to pay for pharmacogenomic testing for dozens of patients.
Insurance coverage remains patchy. Medicare covers some pharmacogenomic testing but not preemptive panels. Private insurers vary wildly in their policies. Some cover testing only after a patient has already experienced an adverse reaction, which defeats much of the purpose. Others refuse coverage entirely, leaving patients to pay out of pocket or forgo testing.
Reimbursement navigation has become complex enough that companies now specialize in helping physicians get pharmacogenomic tests covered. The American Pharmacogenomics Association advocates for broader coverage, arguing that upfront testing costs far less than treating preventable adverse reactions.

The battle over coverage highlights a familiar tension in American healthcare: we know what works, we can calculate that it saves money, but implementation happens slowly and unevenly across different insurance plans and healthcare systems.
The promise of personalized medicine comes with a catch that researchers and clinicians are only beginning to grapple with: most pharmacogenomic research has focused overwhelmingly on people of European ancestry.
This creates real problems. Genetic variants affecting drug metabolism vary significantly across populations. The CYP2D6 gene alone has over 100 known variants, and their frequencies differ dramatically between ethnic groups. Guidelines developed primarily from European genetic data might not apply equally well to Asian, African, or Hispanic patients.
Hmong populations in the United States, for instance, show distinct pharmacogenomic profiles that weren't captured in early research. When healthcare systems implement testing programs based on incomplete data, they risk creating a new kind of health disparity where precision medicine works better for some patients than others.
The FDA has begun requiring pharmaceutical companies to include more diverse populations in clinical trials, but catching up will take years. Meanwhile, the same communities often underserved by traditional medicine face additional barriers to accessing pharmacogenomic testing.
Cost remains an obstacle, obviously. Without consistent insurance coverage, testing becomes another healthcare service available primarily to those who can afford to pay. Community health centers serving low-income populations struggle to offer pharmacogenomic testing even when they recognize its value.
Geography matters too. Academic medical centers in major cities have raced ahead with implementation, but rural hospitals and small clinics lag behind. The infrastructure requirements aren't trivial - you need laboratory partnerships, trained pharmacists or genetic counselors to interpret results, and electronic health record systems sophisticated enough to integrate genetic data into prescribing workflows.
"We risk creating a new kind of health disparity where precision medicine works better for some patients than others."
— Pharmacogenomics equity researchers
Some healthcare systems have found creative solutions. The Sanford Health Imagenetics program in rural South Dakota offers preemptive testing and delivers results through an online portal, making precision medicine accessible beyond urban centers. But these remain exceptions rather than the rule.
Pharmacogenomic testing exists in regulatory gray areas that confuse physicians and patients alike. The FDA regulates some tests as medical devices requiring premarket approval. Others fall under laboratory-developed tests that face lighter oversight. Direct-to-consumer genetic testing companies offer pharmacogenomic information alongside ancestry results, but with variable accuracy and without physician oversight.

Clinical implementation faces challenges beyond regulation. Physicians need education to interpret genetic results and adjust prescriptions accordingly. Many medical schools only recently added pharmacogenomics to their curricula, leaving practicing physicians to learn on the job or through continuing education.
Electronic health record systems, designed before pharmacogenomics became practical, struggle to incorporate genetic data in ways that help rather than overwhelm clinicians. The best systems provide decision support - when a doctor prescribes a medication, the system checks the patient's genetic profile and offers specific recommendations. Less sophisticated approaches bury genetic results in patient charts where they're easily overlooked.
Liability concerns bubble beneath the surface. If a patient suffers an adverse reaction to a drug that genetic testing might have predicted, does the physician bear responsibility for not ordering the test? What about healthcare systems that could implement preemptive testing programs but choose not to because of cost concerns? These questions haven't been definitively answered in court, creating uncertainty for risk-averse institutions.
Technology companies see opportunity in pharmacogenomics, naturally. Artificial intelligence and machine learning promise to find patterns in genetic data that humans might miss. Recent research shows that algorithms analyzing interactions between multiple genes can predict adverse drug reactions with over 90% accuracy, better than looking at individual genes in isolation.
These tools could help physicians navigate the complexity of prescribing for patients with unusual genetic profiles or those taking multiple medications. The average Medicare beneficiary takes four or more prescription drugs; understanding how genetic variants affect each medication and their interactions exceeds human cognitive capacity without computational help.
But algorithms trained on incomplete data inherit the same biases as earlier research. If your training data comes primarily from European populations, your AI will work best for European patients. Companies developing these tools face pressure to ensure their algorithms perform equitably across diverse populations.
The integration challenge extends beyond individual physician practices. Health systems implementing pharmacogenomics need to coordinate laboratories, electronic health records, clinical decision support, pharmacy systems, and physician education programs. Each component must work reliably, and they must work together. Early adopters spent years building this infrastructure; newer programs benefit from their experience but still face significant implementation hurdles.
Pharmacogenomics raises questions that extend beyond medicine into social territory. As genetic testing becomes routine, we need to think carefully about privacy, discrimination, and the potential for genetic information to be misused.
The Genetic Information Nondiscrimination Act provides some protections, prohibiting health insurers and employers from discriminating based on genetic information. But gaps remain - life insurance, disability insurance, and long-term care insurance aren't covered. Some people hesitate to pursue pharmacogenomic testing because they fear genetic information could be used against them later.
As we learn more about genetics, today's curiosity could become tomorrow's crucial prescribing information. Who maintains and updates this data over a patient's lifetime?
Race-based prescribing presents another thorny issue. Some drugs carry dosing recommendations that vary by race, but race is a social construct that correlates imperfectly with genetic ancestry. Pharmacogenomics offers a path forward by focusing on actual genetic variants rather than rough proxies, but the transition will require confronting medicine's complicated history with race.
There's also the question of what counts as actionable information. Your genome contains thousands of variants, most of unknown significance. As we learn more, today's curiosity could become tomorrow's crucial prescribing information. Who maintains and updates this data over a patient's lifetime? How do we notify people when new discoveries make their old genetic test results newly relevant?
The Clinical Pharmacogenetics Implementation Consortium updates guidelines regularly as evidence accumulates, but translating new guidelines into clinical practice happens slowly. A patient tested five years ago might have results that don't reflect current knowledge. Building systems to handle these updates and re-interpretations requires significant infrastructure.
Pharmacogenomics will influence drug development itself. Pharmaceutical companies increasingly use genetic information to stratify clinical trial participants, identifying subgroups likely to benefit from experimental medications. This approach can rescue drugs that failed in broader trials because they work brilliantly for genetically-defined subpopulations.
Cancer treatment has already shifted toward this model. Targeted therapies work in patients whose tumors carry specific genetic markers. The same logic could extend to other conditions. Why develop one antidepressant for everyone when you could develop several, each optimized for different genetic profiles?
This strategy creates economic challenges. Smaller patient populations mean smaller markets, which affects pharmaceutical company incentives. Regulatory pathways designed for blockbuster drugs serving millions don't quite fit medications intended for genetically-defined groups of thousands. The FDA and other regulatory agencies are adapting, but the transition remains incomplete.
Future directions point toward integration with other forms of personalized medicine. Combining pharmacogenomic data with information from wearable devices, electronic health records, and even microbiome analysis could enable truly individualized treatment plans. Your smart watch tracks how you respond to a medication in real-time, your genes predict how you'll metabolize it, your health record provides context, and an AI synthesizes everything into recommendations.
We're also likely to see expansion beyond the roughly 80 gene-drug pairs with current clinical guidelines. Thousands of genetic variants affect drug response; we've only scratched the surface of what's knowable. As genomic sequencing becomes cheaper and faster, comprehensive panels testing hundreds of relevant variants could become standard.
The technology for rapid genome sequencing already exists. Complete genome sequencing now costs under $1,000, less than many specialized pharmacogenomic panels. The bottleneck isn't data generation but interpretation. We need better algorithms, more research, and clearer guidelines to translate genomic information into clinical decisions.
So what should you do with this information? If you take multiple medications, especially cardiovascular drugs, psychiatric medications, or pain relievers, asking your physician about pharmacogenomic testing makes sense. Many doctors remain unaware of testing options or unclear about when to order them, so patient advocacy matters.
If you've experienced unusual reactions to medications - severe side effects at normal doses, or no effect when drugs should have worked - genetic variants might explain why. Pharmacogenetic testing could prevent similar problems in the future.
For people with family histories of adverse drug reactions, preemptive testing offers value even before you need the medications involved. Genetic results don't change over your lifetime, so testing once provides information useful for decades of healthcare decisions.
The state of insurance coverage means you might face out-of-pocket costs. Basic panels testing the most important pharmacogenes cost less than many people assume - often comparable to other routine medical tests. If cost concerns you, ask the testing laboratory about payment plans or patient assistance programs.
Keep in mind that pharmacogenomics isn't fortune-telling. Genetic testing provides probability estimates and guidance, not absolute predictions. Environmental factors, other medications, age, kidney and liver function, and dozens of other variables affect how drugs work in your body. Genetics matter enormously, but they're one piece of a complex puzzle.
The transformation pharmacogenomics represents extends beyond preventing adverse reactions. It signals a fundamental shift in how we think about medicine - from one-size-fits-all to individually tailored, from reactive treatment to proactive prevention, from guessing to knowing.
Healthcare has operated for decades on population averages. We prescribe standard doses because they work for most people, then adjust when they don't. Pharmacogenomics flips this model. We can increasingly predict individual responses before anyone swallows a pill, prescribing the right drug at the right dose the first time.
This matters profoundly for conditions like depression, where patients often suffer through months of failed medications before finding one that helps. It matters for cancer patients whose time is precious and whose bodies can't tolerate unnecessary toxic treatments. It matters for elderly patients taking multiple medications, where adverse drug interactions and reactions send thousands to emergency rooms daily.
The barriers to widespread implementation are real but surmountable. We need better insurance coverage, more physician education, improved clinical decision support systems, and continued research to fill gaps in our knowledge. We particularly need research including diverse populations so that precision medicine delivers on its promise for everyone, not just those whose genetics match historical research subjects.
Progress is accelerating. Five years ago, pharmacogenomic testing was rare outside research hospitals. Today, major healthcare systems integrate it into routine care. Five years from now, genetic-guided prescribing could be standard practice for many common medications. Ten years from now, we might look back and wonder how we ever prescribed drugs without knowing patients' genetic profiles first.
The silent epidemic of adverse drug reactions doesn't need to claim 128,000 American lives annually. The tools to prevent much of this harm already exist in laboratories across the country. The challenge now is implementation - building the systems, training the physicians, educating the patients, and convincing payers that preventing disasters costs less than treating them.
Your DNA has always influenced how medications affect you. The difference now is that we can read those genetic instructions and use them to keep you safer. That's not revolutionary - it's evolution in how we practice medicine, driven by technology that makes the invisible visible and the unpredictable predictable.
The prescription bottle hasn't changed much in decades. What's changing is our ability to know, before anyone opens it, whether the medication inside will help or harm. That knowledge could save your life.
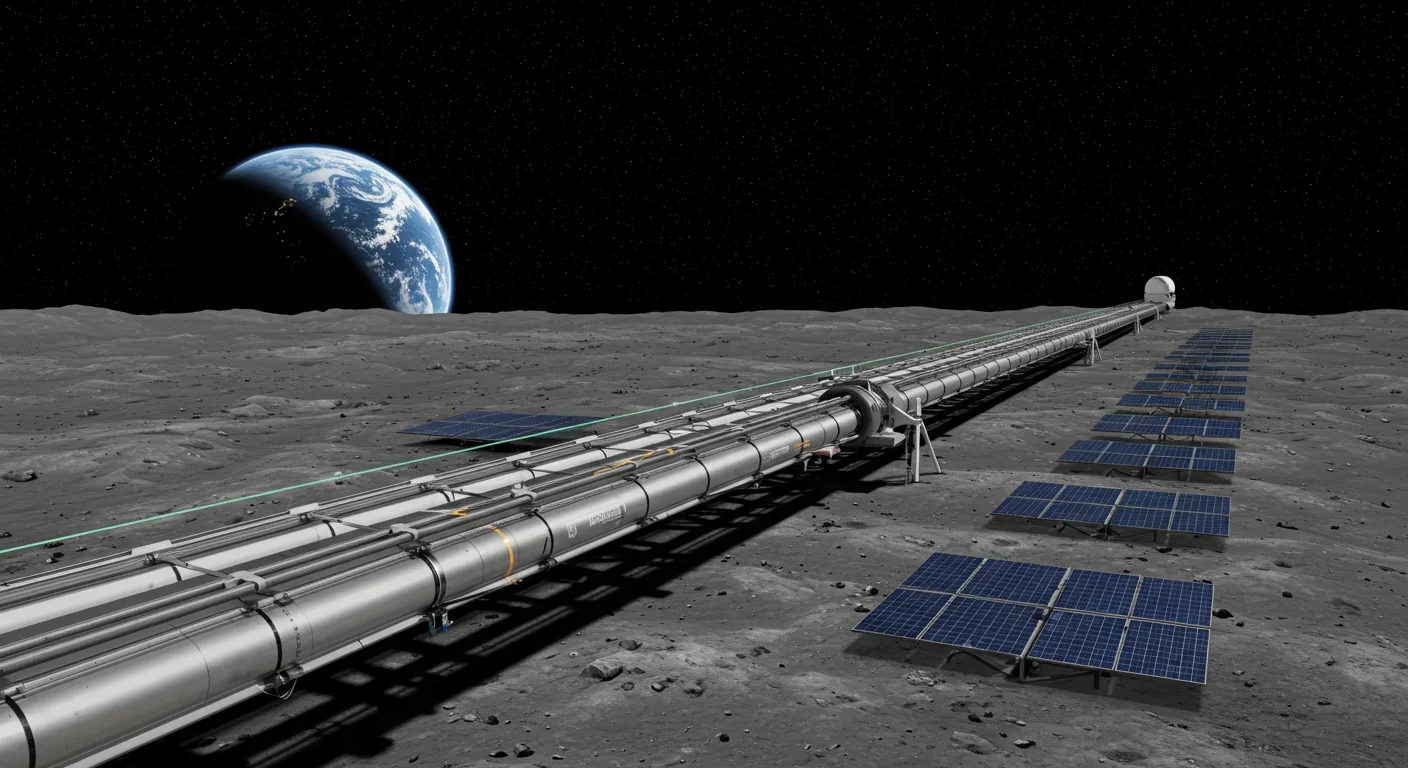
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.