Ancient Archaea in Your Gut: Rewriting Human Biology

TL;DR: Controlled exposure to mild stressors triggers cellular defense mechanisms that extend lifespan—a biological phenomenon called hormesis that explains why practices from intermittent fasting to sauna use share the same longevity-promoting foundation.
What if the secret to living longer isn't avoiding stress but embracing the right kind, in the right doses? Scientists studying aging have uncovered a biological paradox: controlled exposure to mild toxins, stressors, and metabolic challenges can actually extend lifespan and improve health. This counterintuitive phenomenon, called hormesis, rewrites our understanding of longevity and explains why practices as diverse as intermittent fasting, sauna bathing, and eating bitter vegetables all seem to work through the same fundamental mechanism.
The biological principle is elegantly simple: low doses of stressors that would be harmful at high levels instead trigger beneficial adaptive responses. Your cells, honed by millions of years of evolution, respond to mild challenges by upregulating protective pathways that make you more resilient. It's molecular training—what doesn't kill you genuinely makes you stronger, provided the dose is right.

Hormesis describes a two-phased dose-response relationship. At low doses, a substance or stressor produces beneficial effects. At high doses, the same stimulus becomes inhibitory or toxic. Plot this on a graph and you get a distinctive U-shaped or inverted-U curve, depending on what you're measuring.
This isn't theoretical abstraction. In 2012, UCLA researchers found that tiny amounts of ethanol—just 0.005%—doubled the lifespan of starved roundworms. Higher doses of 0.4% provided no longevity benefit. The dose makes the poison, as Paracelsus observed in the 16th century, but it also makes the medicine.
The hormetic curve challenges conventional toxicology: low doses of many substances can be beneficial, while high doses of the same substance become harmful—creating a distinctive U-shaped response pattern that appears across species and stressors.
The hormetic curve challenges the linear-no-threshold models that dominate toxicology and radiation safety policy. Low doses of ionizing radiation, for instance, have been associated with lower cancer mortality in some populations compared to those with no exposure, while high doses dramatically shorten lifespan. This doesn't mean you should seek out radiation, but it illustrates how organisms have evolved sophisticated responses to environmental challenges.
Understanding dose-response relationships is crucial for anyone trying to apply hormetic principles. Too little stress provides no benefit. Too much overwhelms your adaptive capacity. The sweet spot lies in between, and that optimal dose varies by individual, age, health status, and the specific stressor involved.

When you expose your cells to mild stress, they activate an ancient molecular toolkit designed to protect and repair. These stress response pathways overlap and communicate, creating a coordinated defense that extends beyond the initial challenge.
Heat shock proteins (HSPs) are perhaps the most well-studied stress response. These molecular chaperones help other proteins fold correctly, prevent aggregation, and target damaged proteins for degradation. Heat exposure—whether from fever, sauna, or exercise—triggers HSP production. But HSPs respond to more than heat; oxidative stress, heavy metals, and nutrient deprivation also induce them. Small heat shock proteins, in particular, ameliorate protein aggregation linked to neurodegenerative diseases and aging.
When you sit in a sauna at 80-100°C for 20 minutes, you're not just sweating out toxins (you're not, actually—that's mostly a myth). You're triggering heat shock factor 1 (HSF1), which binds to heat shock elements in DNA and cranks up HSP production. Regular sauna use has been associated with reduced cardiovascular mortality and dementia risk, likely mediated through this HSP upregulation.
The Nrf2 pathway governs your antioxidant defenses. Under normal conditions, the protein Keap1 keeps Nrf2 inactive in the cytoplasm, marking it for degradation every 20 minutes. But when oxidative stress or electrophilic compounds modify critical cysteine residues on Keap1, Nrf2 escapes into the nucleus and activates hundreds of protective genes.
"Under oxidative stress, Nrf2 is not degraded, but instead travels to the nucleus where it binds to DNA promoters and initiates transcription of antioxidative genes."
— Research on NFE2L2 pathway mechanisms
These Nrf2-activated genes encode antioxidant enzymes like superoxide dismutase, catalase, and glutathione peroxidase, detoxification enzymes, and proteins involved in DNA repair and autophagy. Remarkably, Nrf2 regulates its own repressor, Keap1, creating a negative feedback loop that prevents overstimulation. Dimethyl fumarate, an FDA-approved multiple sclerosis drug marketed as Tecfidera, works by activating this Nrf2 pathway.
AMPK (AMP-activated protein kinase) acts as your cellular energy sensor. When energy levels drop—during exercise, fasting, or caloric restriction—the ratio of AMP to ATP rises, activating AMPK. This master metabolic regulator stimulates processes that generate energy while inhibiting energy-consuming anabolic pathways.
AMPK activation promotes autophagy, mitochondrial biogenesis, glucose uptake, and fatty acid oxidation. It inhibits mTOR, a growth-promoting pathway whose chronic activation is associated with aging and cancer. The diabetic drug metformin, which many longevity enthusiasts take off-label, works primarily through AMPK activation.

Sirtuins are NAD+-dependent deacetylases that regulate metabolism, stress resistance, and longevity. Caloric restriction and fasting increase NAD+ levels, activating sirtuins. These proteins deacetylate histones and transcription factors, influencing gene expression patterns that promote survival during nutritional stress.
SIRT1, the best-studied sirtuin, enhances insulin sensitivity, promotes fat mobilization, improves mitochondrial function, and extends lifespan in yeast, worms, and flies. Whether sirtuins mediate lifespan extension in mammals remains debated, but they clearly orchestrate metabolic adaptation to energy scarcity.
Autophagy—literally "self-eating"—is your cellular recycling program. During autophagy, cells engulf damaged organelles, misfolded proteins, and pathogens in double-membrane vesicles called autophagosomes, which fuse with lysosomes for degradation. The breakdown products get reused as building blocks and fuel.
Fasting, exercise, and caloric restriction all stimulate autophagy. This process declines with age, and impaired autophagy is implicated in neurodegenerative diseases, cancer, and metabolic disorders. Rapamycin, which inhibits mTOR and promotes autophagy, extends lifespan in mice, but at doses that must be carefully calibrated—too much completely shuts down mTOR, which is toxic.
Mitochondria, the powerhouses generating ATP through oxidative phosphorylation, are also major producers of reactive oxygen species (ROS). For decades, ROS were viewed as purely damaging—the toxic byproducts of metabolism that accumulate with age and cause cellular dysfunction.
The mitochondrial free radical theory of aging posited that ROS damage accumulates over time, degrading proteins, lipids, and DNA until cells can no longer function. Antioxidant supplementation seemed like an obvious intervention. But clinical trials of antioxidant vitamins have been disappointing, sometimes even harmful.
The failure of antioxidant supplements revealed a critical insight: low levels of reactive oxygen species aren't cellular damage—they're signaling molecules that trigger protective adaptations. Blocking them with antioxidants may prevent the very adaptations that extend lifespan.
Enter mitohormesis: the idea that low levels of mitochondrial ROS act as signaling molecules that trigger adaptive responses, improving mitochondrial function and extending lifespan. Mild mitochondrial stress—from exercise, caloric restriction, or polyphenols—generates a ROS burst that activates protective pathways.
This ROS signaling activates transcription factors like Nrf2 and promotes mitochondrial biogenesis through PGC-1α. The result? More efficient, healthier mitochondria and upregulated antioxidant defenses. Red light therapy exemplifies this principle: near-infrared light stimulates cytochrome c oxidase in mitochondria, producing a transient ROS increase that primes antioxidant systems.
When researchers inhibit mitochondrial ROS production during exercise or caloric restriction, the lifespan-extending benefits disappear. The signaling matters. This explains why flooding your system with antioxidant supplements can backfire—you're blocking the very stress signals that trigger adaptation.

Caloric restriction is the most robust longevity intervention across species. Reducing calorie intake by 20-40% without malnutrition extends lifespan in yeast, worms, flies, rodents, and possibly primates. The mechanism involves activation of AMPK, sirtuins, autophagy, and reduced mTOR signaling—the entire hormetic stress response suite.
But caloric restriction is difficult for humans to sustain. It can reduce bone density, libido, and cold tolerance. Some researchers argue that the benefits in rodents may not translate to humans, who evolved with more variable food availability. Intermittent fasting and time-restricted eating may capture some hormetic benefits with better adherence.
Exercise is hormesis in action. During intense physical activity, you generate oxidative stress, deplete energy stores, damage muscle fibers, and stress your cardiovascular system. Your body responds by building stronger mitochondria, increasing antioxidant capacity, and improving metabolic flexibility.
Moderate, regular exercise reduces disease risk across the board. But the relationship follows a hormetic curve: sedentary individuals have high disease risk, moderate exercisers have the lowest risk, and extreme endurance athletes may see some of those benefits plateau or even reverse due to chronic overtraining stress. The key is finding your personal hormetic zone where stress and recovery balance.
"Physical exercise intensity may exhibit a hormetic curve. Individuals with low levels of physical activity are at risk for some diseases; however, individuals engaged in moderate, regular exercise may experience less disease risk."
— Research on exercise hormesis, Wikipedia
Polyphenols from plants—resveratrol in grapes, EGCG in green tea, curcumin in turmeric, sulforaphane in broccoli—are often called antioxidants, but that's misleading. Most polyphenols are weak pro-oxidants that generate mild oxidative stress, activating Nrf2 and other protective pathways.
This phenomenon is called xenohormesis: organisms benefit from stress response compounds that plants produce when they're under environmental pressure. When you eat stressed plants—plants exposed to drought, UV radiation, or pathogens—you ingest their defensive chemicals, which trigger your own stress responses.
Sulforaphane from cruciferous vegetables is among the most potent Nrf2 activators. It's produced when the enzyme myrosinase acts on glucoraphanin, which happens when you chew broccoli. Cooking destroys myrosinase, so lightly steaming broccoli and adding mustard powder (which contains myrosinase) maximizes sulforaphane availability. This compound has shown neuroprotective effects in animal models of Alzheimer's and Parkinson's.

Temperature stress provides another hormetic avenue. Cold exposure—whether through cold showers, ice baths, or winter swimming—activates brown adipose tissue, which generates heat by uncoupling mitochondrial respiration. This thermogenic process produces ROS and activates stress response pathways similar to exercise.
Regular cold exposure improves insulin sensitivity, increases brown fat activity, and may enhance immune function. Some studies suggest it activates autophagy and extends lifespan in animal models. The key is brief, intense cold exposure—enough to trigger adaptation without inducing dangerous hypothermia.
Heat stress, beyond triggering heat shock proteins, also activates FOXO transcription factors that promote longevity. Finnish studies show that men who use saunas 4-7 times per week have significantly lower risks of cardiovascular death and dementia compared to those who use them once weekly. The dose-response is clear.
Why would organisms respond to mild stress by becoming more resilient? From an evolutionary perspective, it makes perfect sense. Environments fluctuate. Resources vary. Organisms that can anticipate future challenges based on current mild stressors gain a survival advantage.
If a plant experiences mild drought today, it's likely to face more severe drought soon. Upregulating stress defenses preemptively is adaptive. Similarly, if an animal experiences food scarcity, preparing for prolonged famine by enhancing metabolic efficiency and cellular repair increases survival probability.
Hormesis represents this predictive adaptation. The stressor acts as an informational cue, prompting the organism to fortify its defenses before a more severe challenge arrives. Over evolutionary time, organisms optimized these responses, developing sensitive dose-detection mechanisms that distinguish hormetic signals from toxic insults.
The historical practice of mithridatism—named after King Mithridates VI of Pontus, who allegedly built immunity to poisons through gradual exposure—demonstrates ancient empirical understanding of hormesis. Modern toxicology initially dismissed such claims, but the hormetic framework rehabilitates them with mechanistic rigor.
The hormetic principle offers a unifying framework for understanding diverse longevity interventions, but translating research findings into personal practice involves uncertainties. Individual variation in stress tolerance, baseline health, genetics, and lifestyle factors all influence optimal dosing.
The critical difference between beneficial hormetic stress and harmful chronic stress lies in intensity, duration, and recovery. Hormetic stressors are acute and intermittent, followed by recovery periods when adaptation occurs. Chronic unrelenting stress depletes adaptive capacity and accelerates aging.
What works: Incorporating hormetic stressors through lifestyle rather than supplements appears most effective. Regular moderate exercise, occasional fasting or time-restricted eating, sauna use, and consuming a variety of colorful vegetables provide multiple hormetic signals without requiring precise dosing.
What's unclear: Optimal frequencies and intensities for different populations. An intervention that's hormetic for a healthy 30-year-old might be overwhelming for a frail 80-year-old. Age, sex, baseline fitness, and disease status all modify the hormetic dose-response curve.
What doesn't work: Attempting to bypass the stress with antioxidant supplements. High-dose vitamin E, beta-carotene, and other antioxidants have failed to extend lifespan in clinical trials and may interfere with beneficial hormetic signaling. The stress matters—you can't shortcut adaptation.
Emerging approaches: Researchers are developing hormetin drugs—pharmaceuticals that mimic hormetic stress responses without requiring actual stressors. Metformin and rapamycin analogs (rapalogs) are candidates, but long-term human data remains limited. NAD+ precursors like NMN and NR aim to boost sirtuin activity, though their efficacy and safety at high doses is still debated.
Potential risks: Applying hormesis incorrectly can cause harm. Chronic stress—whether psychological, physical, or metabolic—is the opposite of hormetic stress. The critical difference lies in intensity, duration, and recovery. Hormetic stressors are acute and intermittent, followed by recovery periods when adaptation occurs. Chronic unrelenting stress depletes adaptive capacity and accelerates aging.
Some critics, like bioenergetic health practitioner Jay Feldman, argue that hormesis research is often flawed, confounding stress with correction of underlying deficiencies. For example, does caloric restriction extend lifespan because mild stress is beneficial, or because it corrects the harmful effects of overfeeding laboratory animals? These questions matter for human application.
As our understanding of stress response pathways deepens, the hormetic framework is reshaping how we think about aging, disease prevention, and therapeutic interventions. Rather than viewing aging as inevitable decline, hormesis suggests we can actively train our cells to be more resilient.
Biomarkers for hormetic stress—measuring HSP levels, Nrf2 activation, autophagy flux, or mitochondrial function—could help personalize interventions. Wearable devices might eventually track stress-recovery cycles, optimizing your hormetic dose in real-time.
The concept also challenges medical paradigms that pathologize all stress. Fever, for instance, activates immune responses and may shorten illness duration. Aggressively suppressing fever with antipyretics might interfere with this adaptive response. Similarly, inflammation after exercise is necessary for muscle adaptation; excessive anti-inflammatory use could blunt training benefits.
Clinical trials are now investigating hormetic interventions systematically. Studies on intermittent fasting, heat therapy, and cold exposure are moving beyond animal models to human longevity outcomes. The next decade should clarify which hormetic practices have the strongest evidence base and how to optimize them for different populations.
Perhaps most importantly, hormesis provides a biological foundation for ancient wisdom about moderation, challenge, and resilience. The Stoics understood that voluntary discomfort builds character. Hormesis explains how it also builds cellular resilience at the molecular level.
The paradox of poison resolves into a more nuanced understanding: the biological systems that keep you alive thrive on challenge, but only in the right doses and contexts. Your cells evolved to respond to intermittent stress with adaptive fortification. Avoiding all stress isn't optimal—it leaves those systems under-stimulated and under-prepared.
The hormetic sweet spot lies between comfort and overwhelm. You can access it through practices that our ancestors encountered naturally: periods without food, temperature extremes, physical exertion, and consuming plants stressed by their own environments. Modern life insulates us from many of these stressors, potentially depriving our cells of the very signals they need to maintain resilience.
Understanding hormesis empowers you to be strategic about stress. Not all stress is created equal. Chronic psychological stress from work deadlines differs fundamentally from the acute metabolic stress of a workout or fast. The former depletes you. The latter, dosed correctly and followed by recovery, makes you stronger.
The evidence suggests that longevity isn't about achieving perfect homeostasis or eliminating all oxidative stress. It's about maintaining dynamic resilience—the capacity to respond, adapt, and recover from challenges. Hormesis is the mechanism. The dose makes the medicine. And what doesn't kill you, if carefully calibrated, genuinely makes you stronger at the cellular level, extending both your lifespan and the quality of the years you live.
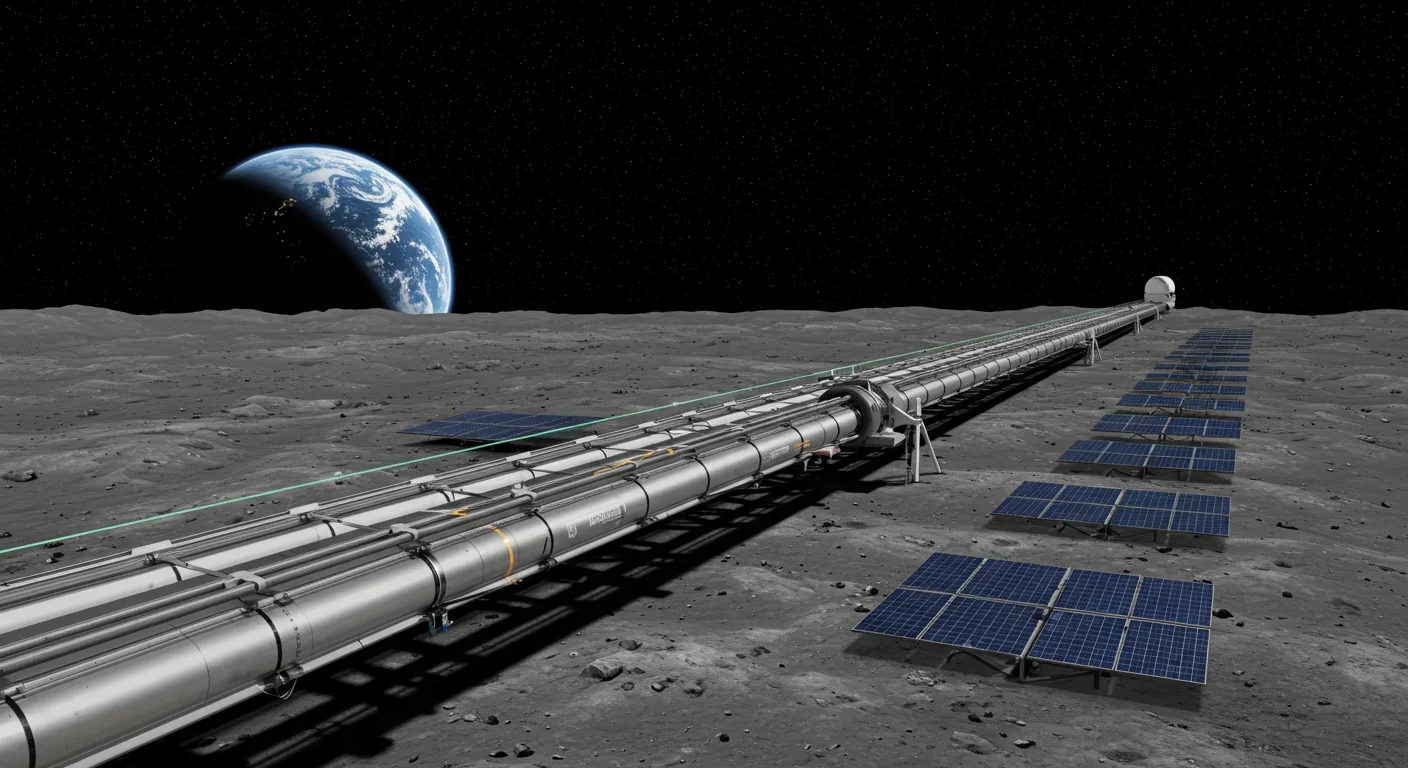
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.