How Soft Corals Weaponize Their Symbiotic Algae

TL;DR: Sea slugs perform one of nature's most audacious heists: stealing chloroplasts from algae and maintaining them inside their own cells to photosynthesize, surviving months without eating while running on stolen solar power.
Picture an animal that looks like a bright green leaf, drifting through shallow coastal waters. It doesn't eat for months but somehow survives. The secret? It's running on stolen solar panels—chloroplasts plucked from algae and woven into its own body. Welcome to the bizarre world of photosynthetic sea slugs, creatures that challenge everything we thought we knew about the boundaries between plant and animal life.

These remarkable organisms practice what scientists call kleptoplasty—literally "stolen plastids"—a biological heist so audacious it sounds like science fiction. They pierce algae cells, suck out the chloroplasts (the organelles that perform photosynthesis), and incorporate them into their own digestive tissue where they continue producing energy from sunlight. The result: animals that are genuinely solar-powered, existing in a twilight zone between kingdoms of life.
The champion of this peculiar adaptation is Elysia chlorotica, a species found along the Atlantic coast of North America. As juveniles, these sea slugs start out gray-brown and unremarkable. But after feeding on their preferred algae, Vaucheria litorea, they transform into vibrant, leaf-like creatures measuring up to six centimeters long.
The transformation happens at the cellular level. When E. chlorotica pierces algae cells with its specialized radula (a razor-sharp feeding structure), it doesn't digest everything. Instead, it selectively diverts chloroplasts into intestinal sacs where they're encased in a specialized membrane that researchers call kleptosomes—essentially biological storage units for stolen goods.
Chloroplasts continue to photosynthesize inside animal cells for up to ten months, converting sunlight into energy exactly as they would inside a plant—a feat that shouldn't be biologically possible.
Here's where it gets truly extraordinary: these chloroplasts continue to photosynthesize inside animal cells, converting sunlight into energy exactly as they would inside a plant. The slugs store the chloroplasts in the extensive branching tubes of their digestive system, creating a distributed solar array throughout their bodies. According to research published in the journal Cell, well-fed slugs turn brilliant green as their bodies fill with functional chloroplasts, while starved ones gradually turn orange as the chloroplasts degrade—just like leaves changing color in autumn.
But keeping plant machinery running inside animal cells shouldn't be possible. Chloroplasts are notoriously fragile outside their natural environment, typically breaking down within hours or days. So how do these slugs maintain them for months?

The key lies in what the slugs don't do. Most animal cells would quickly destroy foreign organelles using lysosomes—cellular structures that function as biological trash disposals, breaking down damaged or unwanted material. But researchers led by Corey Allard at Harvard discovered that Elysia crispata (a closely related species) has evolved the ability to downregulate these lysosomes, essentially turning down the volume on their cellular cleanup systems.
Chemical analysis revealed another surprise: the stolen chloroplasts contained slug proteins, not just algal ones. This means the slugs aren't just passively storing the chloroplasts—they're actively maintaining and possibly repairing them. The host animal is somehow supporting the continued function of these hijacked organelles, providing protective proteins that keep them healthy.
This maintenance system allows some species like E. chlorotica to survive for nine to ten months without eating, relying entirely on the sugars produced by their internal chloroplasts. That's like going without food for three-quarters of a year while your stolen solar panels keep you alive.
"This is an organism that can steal parts of other organisms, put them in their own cells, and use them."
— Corey Allard, Harvard University
The evolutionary implications are staggering. Chloroplasts themselves originated through endosymbiosis—ancient bacteria that were engulfed by primitive cells and never left, eventually becoming permanent residents. What we're witnessing in these sea slugs might be evolution experimenting with a similar process, though on a much faster timescale.
For years, one of the most controversial questions in this field has been whether the slugs have stolen not just chloroplasts, but also genes from their algal prey. In 2008, researchers reported finding psbO—a vital algal gene involved in photosynthesis—in the DNA of E. chlorotica, suggesting horizontal gene transfer between different kingdoms of life.

If true, this would mean the slugs had permanently incorporated algal genetic material into their own genomes, potentially passing it to their offspring. It would be one of the clearest examples of horizontal gene transfer in complex animals—a phenomenon more commonly associated with bacteria swapping antibiotic resistance genes.
But the story isn't that simple. Later research has cast doubt on these early findings, with some scientists unable to replicate the results. The debate continues, with researchers divided on whether true genetic integration has occurred or whether the slugs simply maintain chloroplasts through entirely cellular mechanisms.
What's clear is that the chloroplasts themselves come equipped with some of the genes they need to survive. The algae Vaucheria litorea has chloroplasts that produce ftsH, a protein essential for repairing photosystem II—one of the key molecular machines in photosynthesis. This built-in repair capability may explain why these particular chloroplasts can survive so much longer than others when transplanted into animal cells.
Complicating matters further, experiments by German researcher Sven Gould showed that E. chlorotica can survive without light almost as well as slugs exposed to sunlight, even when photosynthesis is blocked. After 55 days without food or light, the slugs were still alive. This suggests that photosynthesis alone might not account for their survival—there may be additional energy sources or metabolic tricks at play.
Elysia chlorotica isn't alone in this solar-powered lifestyle. The phenomenon spans multiple species across the sacoglossan sea slug family, though with varying degrees of success. Elysia timida, found in Mediterranean waters, also practices kleptoplasty but retains functional chloroplasts for shorter periods—typically weeks rather than months.
Plakobranchus ocellatus, another sacoglossan found in Indo-Pacific waters, has evolved its own approach to the lifestyle. These slugs feed on different algal species and have adapted their cellular machinery accordingly. The diversity of kleptoplastic species suggests this adaptation has evolved multiple times independently—a phenomenon called convergent evolution that indicates this is a genuinely beneficial survival strategy.

Even beyond sea slugs, nature has experimented with similar arrangements. The nudibranch Pteraeolidia ianthina sequesters whole living zooxanthellae (photosynthetic dinoflagellates) within its digestive tissue, becoming what researchers call "solar-powered" in its own right. These nudibranchs maintain entire symbiotic organisms rather than just stealing their chloroplasts.
But there's significant variation in how long different species can maintain their stolen solar panels. Rhabdocoel flatworms that practice kleptoplasty retain their chloroplasts for only about seven days, while E. chlorotica holds the record at ten months. This variation reflects different evolutionary solutions to the same challenge: how to keep foreign organelles alive inside your cells.
While photosynthesis gets all the attention, researchers suspect the chloroplasts serve multiple functions beyond energy production. The most obvious alternative benefit is camouflage—the bright green coloration from chloroplasts helps slugs blend perfectly into algae-covered rocks and seagrass beds in their shallow-water habitats, typically at depths of 0 to 0.5 meters where light is abundant.
There's also evidence the chloroplasts might store or produce defensive chemicals. Many algae produce toxic compounds to deter herbivores, and if these chemicals accumulate in the chloroplasts, the slugs would essentially steal both solar power and chemical weapons in one go. This would explain why predators generally avoid these colorful, conspicuous slugs despite their soft, unprotected bodies.
Roughly 150 species across multiple families have independently evolved chloroplast retention, suggesting this adaptation provides significant survival advantages beyond just energy production.
Recent research published in Frontiers in Marine Science found that Elysia crispata secretes mucopolysaccharides (complex sugars) that incorporate carbon from chloroplast photosynthesis. This suggests the stolen chloroplasts are integrated into the slugs' metabolism in ways we're only beginning to understand, potentially contributing to everything from slime production to immune function.

The adaptive value appears to be so significant that roughly 150 species across multiple families have independently evolved some form of chloroplast retention—though sacoglossan sea slugs have taken the strategy to its extreme.
Here's where the story takes an unexpected turn toward human health. The same cellular mechanism that allows sea slugs to keep chloroplasts alive—downregulating lysosomes—might offer insights into treating lysosomal storage disorders, a group of about 50 rare genetic diseases where lysosomes malfunction.
In these conditions, cells can't properly break down certain substances, leading to toxic accumulation that damages organs and nervous tissue. Diseases like Gaucher disease, Fabry disease, and several forms of childhood neurodegeneration fall into this category. Understanding how sea slugs can so precisely control their lysosomal activity might reveal new therapeutic targets.
"This could be applied to neurodegenerative conditions or lysosomal storage disorders," researchers noted in a Harvard Gazette article discussing the implications. The molecular pathways these slugs use to downregulate their cellular cleanup systems might be translatable to medical interventions—a reminder that studying nature's oddities often yields unexpected practical applications.
The ability of sea slugs to maintain functional chloroplasts has inspired an even more ambitious goal: creating animal cells that can photosynthesize from scratch. In a groundbreaking 2024 study, Japanese researchers successfully incorporated chloroplasts into hamster cells in the laboratory—the first time animal cells were made to photosynthesize outside of natural systems.
The implications stretch from lab-grown meat production (imagine culturing tissue that partially feeds itself through photosynthesis, reducing nutrient costs) to new approaches in regenerative medicine and tissue engineering. If we could endow human cells with even a fraction of the photosynthetic capability that sea slugs have mastered, it might revolutionize how we approach tissue transplants or bioengineered organs.
Researchers at the University of Tokyo created these photosynthetic animal cells by isolating chloroplasts from red algae and introducing them into hamster cell cultures. The chloroplasts survived for at least two days and continued producing oxygen—proof of concept that the barrier between animal and plant cellular machinery isn't as absolute as we once believed.
"The organelles continue to perform photosynthesis, providing nutrients and energy to their hosts and serving as emergency rations in times of starvation."
— Harvard Gazette
A European research project called KLEPTOS is taking this further, using kleptoplasty to illuminate the broader evolution of plastids and the sequence of steps during endosymbiosis. By studying how sea slugs accomplish this feat naturally, scientists hope to understand the fundamental principles that could eventually allow us to engineer photosynthetic capabilities into other organisms—or even create artificial cells that combine the best features of both kingdoms.
From an evolutionary perspective, kleptoplasty represents a fascinating middle ground. It's not quite true endosymbiosis (where a permanent merger occurs between two organisms), but it's far more than simple predation. The slugs have evolved specialized cellular machinery to support organelles from an entirely different kingdom of life—without permanently integrating them into their genome.
This raises profound questions about how major evolutionary transitions occur. The chloroplasts in all plants today originated from ancient cyanobacteria that were engulfed by primitive eukaryotic cells roughly 1.5 billion years ago. Over hundreds of millions of years, most of the bacterial genes migrated to the host cell's nucleus, leaving behind the stripped-down chloroplast genomes we see today.
Are sacoglossan sea slugs in the early stages of a similar process? Probably not—they haven't had enough time, and the chloroplasts still degrade eventually. But they demonstrate that evolution can produce intermediate stages between predation and permanent symbiosis, blurring the lines between "eating" and "becoming."
The horizontal gene transfer debate adds another layer of complexity. If genes have indeed transferred from algae to slugs, it would suggest that evolution can sometimes take shortcuts, borrowing genetic innovations from other lineages rather than developing them from scratch through random mutation.
Like many marine organisms, kleptoplastic sea slugs face challenges from changing ocean conditions. Research has shown that water temperature affects how efficiently the chloroplasts photosynthesize inside slug tissue, with both unusually warm and cold temperatures reducing photosynthetic output.
This temperature sensitivity matters because these slugs inhabit shallow coastal zones—exactly the habitats most affected by climate change. Rising sea temperatures, combined with coastal development and pollution, threaten the specific algae species that different slug species depend on. Elysia chlorotica can only steal chloroplasts from Vaucheria litorea, so if that algae species declines or shifts its range, the slugs may face difficulties.
The specialized nature of these relationships makes them particularly vulnerable to disruption. Unlike generalist predators that can switch prey species, kleptoplastic slugs have evolved intricate cellular machinery tuned to specific chloroplasts from specific algae. That specialization, which allows them to maintain chloroplasts for months, also creates dependency.
Perhaps the most profound implication of photosynthetic sea slugs is what they teach us about the flexibility of biological categories. We've long organized life into tidy kingdoms—plants, animals, fungi, bacteria—but nature consistently defies these classifications.
These slugs aren't plants, yet they photosynthesize. They're not algae, yet they contain functional chloroplasts. They're animals that blur into the plant kingdom every time they feed, creating a living mosaic that doesn't fit neatly into textbooks.
This biological ambiguity isn't just a curiosity—it reflects a deeper truth about evolution. Life doesn't respect the boundaries we create for it. Given enough time and the right environmental pressures, organisms will experiment with seemingly impossible adaptations, hijacking cellular machinery from other kingdoms, evolving capabilities that seem to violate the rules.
These sea slugs represent evolution's willingness to experiment with seemingly impossible combinations, demonstrating that the boundaries between kingdoms of life are far more flexible than we imagined.
In an age when we're learning to engineer organisms with unprecedented precision, understanding natural systems like kleptoplasty becomes increasingly important. These sea slugs have already solved problems that bioengineers are just beginning to tackle—how to integrate plant and animal cellular systems, how to maintain foreign organelles, how to create functional hybrids between different forms of life.
The leaf-shaped creatures drifting through shallow coastal waters represent more than just an evolutionary oddity. They're a reminder that life is far more creative, flexible, and surprising than we imagine. They challenge our definitions, inspire our technologies, and demonstrate that even after billions of years of evolution, nature still has new tricks to show us.
What other impossible combinations might be waiting to be discovered in the ocean's depths or in ecosystems we've barely explored? If animals can steal the power of photosynthesis, what other boundaries might be more flexible than we think? The sea slugs that became part-plant suggest that we've only scratched the surface of life's possibilities.
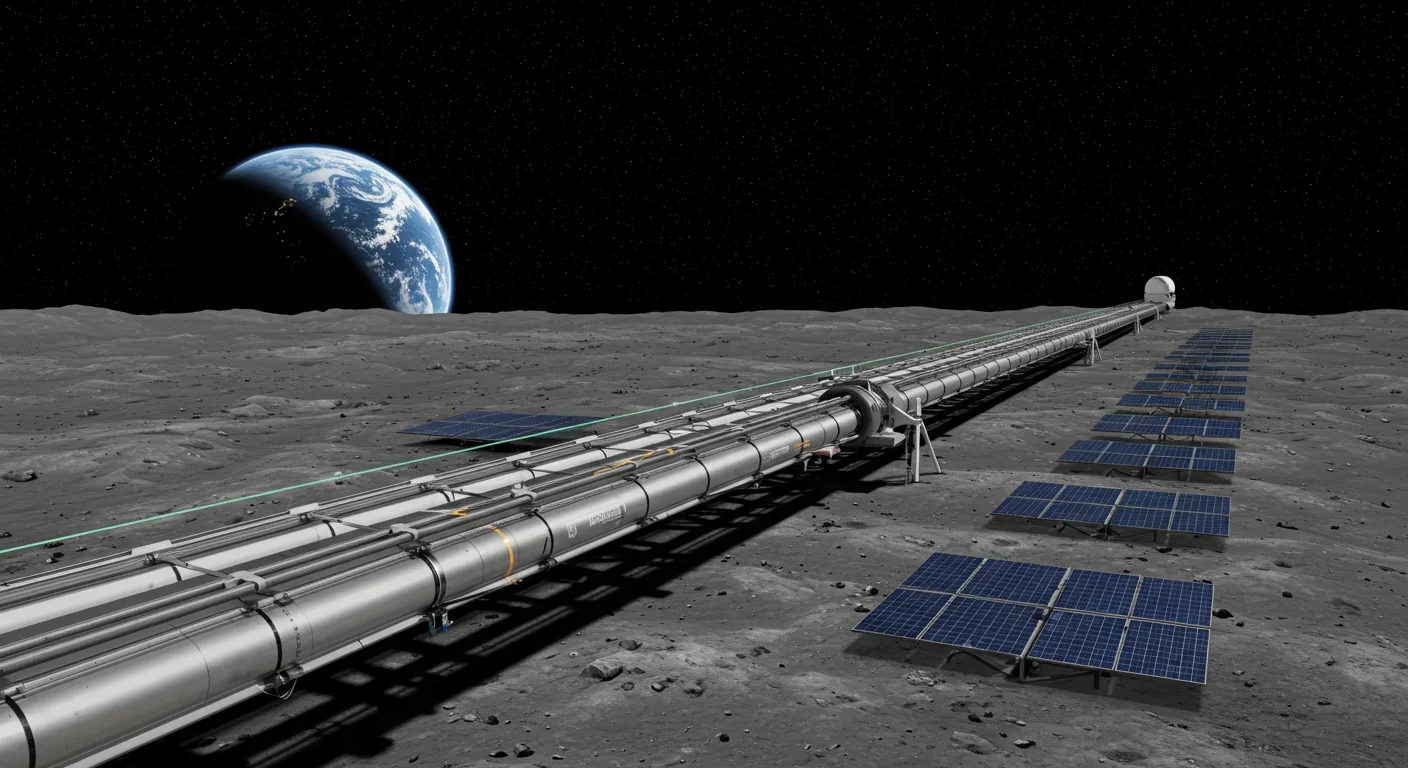
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.