How Soft Corals Weaponize Their Symbiotic Algae

TL;DR: Deep-sea animals like giant tubeworms have evolved to survive without mouths or digestive systems, hosting billions of chemosynthetic bacteria that convert toxic chemicals into nutrition. This remarkable symbiosis thrives in Earth's most extreme environments and reveals new possibilities for life beyond our planet.
Imagine surviving without ever taking a single bite of food. No breakfast, no lunch, no dinner—nothing. For most of us, that's a terrifying thought. But deep beneath the ocean's surface, in some of Earth's most extreme environments, entire communities of animals have evolved to do exactly that. They've abandoned mouths, guts, and traditional digestion altogether, relying instead on microscopic partners that convert toxic chemicals into nutrition.
These aren't science fiction creatures. They're real animals living right now on our planet, in places so hostile that scientists once thought life couldn't exist there at all.

The story begins in 1977, when the research submersible Alvin descended to the Galápagos Rift, a volcanic fracture zone 2,500 meters below the Pacific Ocean. What the crew found changed biology forever. Surrounding superheated vents belching water at temperatures up to 464°C were dense thickets of giant red-plumed worms, some reaching three meters in length.
The scientists were baffled. This deep, no sunlight penetrates. Photosynthesis—the process that powers nearly all life on Earth's surface—is impossible here. Yet these worms, later named Riftia pachyptila, weren't just surviving. They were thriving, growing faster than almost any other marine invertebrate known.
When researchers examined the worms more closely, they discovered something even more astonishing: adult tubeworms have no mouth, no stomach, and no anus. Zero digestive equipment. It was as if someone had designed an animal and forgotten to include the most fundamental system for staying alive.
How could this possibly work?
Giant tubeworms dedicate 40-50% of their body weight to housing bacteria—an investment that enables them to grow up to 1.5 meters in less than two years, making them among the fastest-growing marine invertebrates on Earth.
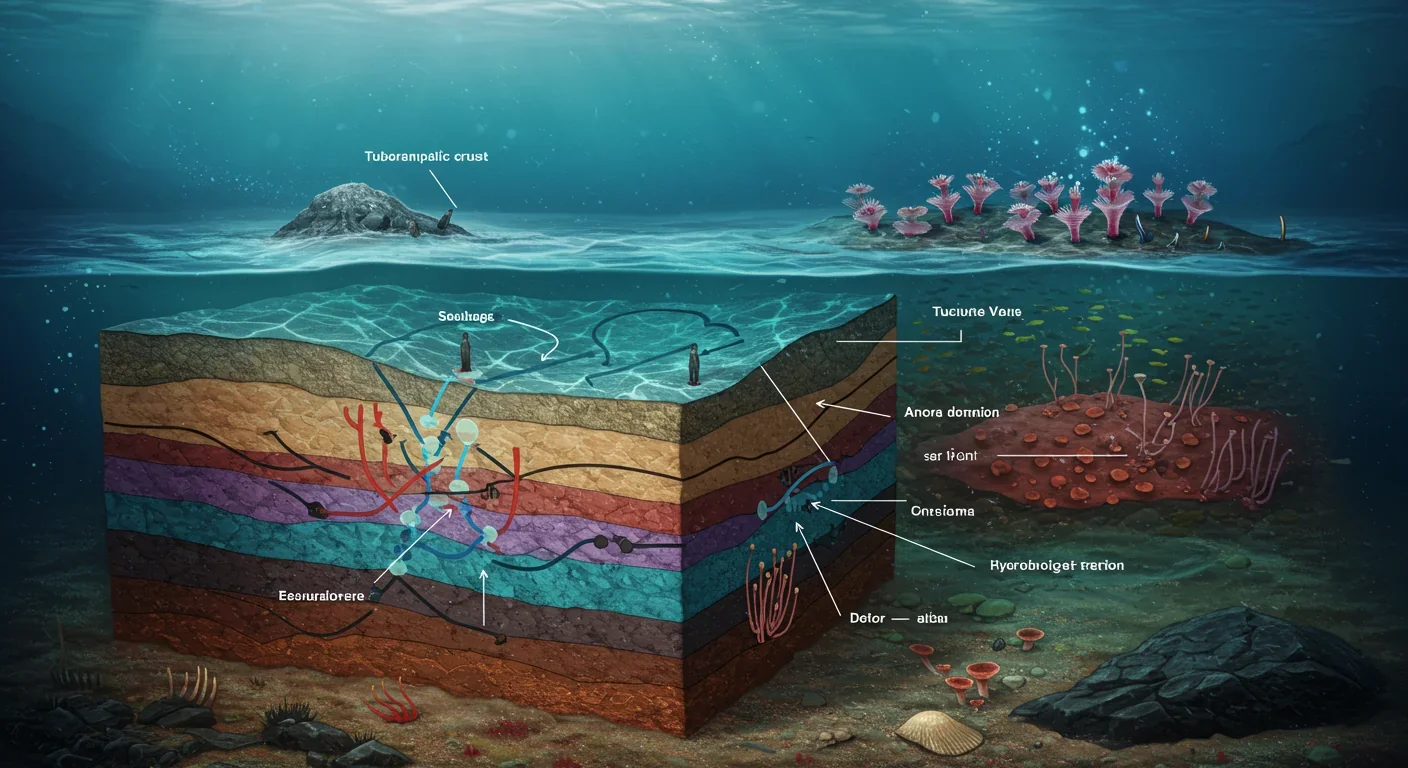
The answer lay hidden inside the worms' bodies. Nearly half of each tubeworm's weight consists of a spongy organ called the trophosome—a specialized tissue packed with billions of chemosynthetic bacteria. These microbes, primarily Candidatus Endoriftia persephone, have essentially become the worm's external digestive system, living inside specialized cells called bacteriocytes.
The bacteria perform a trick that seems almost magical: they take hydrogen sulfide—a compound so toxic it's used as a chemical weapon—and convert it into food. The process, called chemosynthesis, works like photosynthesis's dark twin. Instead of using sunlight to convert carbon dioxide into sugars, these bacteria harness the chemical energy released when they oxidize hydrogen sulfide.

The reaction looks deceptively simple on paper: 18H₂S + 6CO₂ + 3O₂ → C₆H₁₂O₆ + 12H₂O + 18S. Hydrogen sulfide plus carbon dioxide plus oxygen yields glucose, water, and elemental sulfur. But making this work inside a living animal, at crushing ocean depths, in water that can scald or freeze depending on how close you are to the vent, required extraordinary evolutionary innovations.
The tubeworm's contribution to this partnership is equally remarkable. Its bright red plume—the only part visible outside the protective tube—functions as a sophisticated gill. Blood vessels in the plume absorb hydrogen sulfide, carbon dioxide, and oxygen from the surrounding water. Then comes the really clever part: the worm's hemoglobin can bind both oxygen and hydrogen sulfide simultaneously without either interfering with the other, thanks to specialized zinc-coordination sites. This unique molecule safely transports both gases through the worm's bloodstream to the bacteria waiting in the trophosome, preventing sulfide from poisoning the host's own tissues.
The bacteria oxidize the sulfide, fix the carbon dioxide using the Calvin cycle—the same biochemical pathway plants use—and produce organic molecules like sugars and amino acids. The worm then absorbs these nutrients directly from the bacteria. It's a closed-loop system: the worm provides raw materials and a safe home, the bacteria provide all the food.
What makes this story even more fascinating is that giant tubeworms aren't alone. Multiple animal groups have independently evolved similar partnerships, a phenomenon biologists call convergent evolution. When the same solution appears repeatedly in unrelated lineages, it's usually because that solution is extraordinarily effective.
At hydrothermal vents and cold seeps—places where methane and hydrogen sulfide seep from the seafloor—you'll find an entire bestiary of animals that have traded traditional digestion for bacterial partnerships. Deep-sea clams like Calyptogena magnifica and mussels such as Bathymodiolus thermophilus harbor chemosynthetic symbionts in their gills. Some species even host multiple types of bacteria simultaneously: sulfur-oxidizers and methane-oxidizers working side by side.
These animals span multiple phyla—annelids (tubeworms), mollusks (clams, mussels, limpets), and crustaceans (shrimp, crabs)—showing that chemosynthetic symbiosis isn't a one-off quirk but a viable life strategy that evolution has discovered again and again. Each lineage evolved its own specialized housing for bacteria: tubeworms use the trophosome, clams and mussels use modified gill cells, and some shrimp cultivate bacterial gardens on their mouthparts.

"These organisms have become so reliant on their symbionts that they have lost all morphological features relating to ingestion and digestion."
— Wikipedia: Hydrothermal Vent Microbial Communities
The bacterial partners are equally diverse. Most belong to groups called Gammaproteobacteria and Campylobacterota, but recent genomic studies have revealed surprising diversity even among symbionts of closely related hosts. Bacteria from hydrothermal vents show more than 98% genetic similarity to each other, while those from cold seeps can differ by more than 7%—suggesting that habitat type shapes symbiont evolution just as much as host species does.
Here's a puzzle: if adult tubeworms lack any way to eat, how do baby worms acquire their essential bacterial partners in the first place?
The answer reveals another layer of evolutionary sophistication. Tubeworms reproduce by releasing eggs and sperm into the water, where fertilization occurs. The resulting larvae are free-swimming and actually possess temporary digestive structures. When a larva settles near a vent, it ingests bacteria from the surrounding water through a small opening in its developing plume. Once the right bacterial species are established, the larva undergoes a dramatic transformation: its primitive gut dissolves and is replaced by the trophosome, sealing the worm into a lifetime of obligate symbiosis.
This horizontal transmission—acquiring symbionts from the environment rather than inheriting them from parents—appears in many chemosynthetic partnerships. It's risky because larvae must find the right bacteria, but it's also flexible, allowing each generation to potentially acquire locally adapted symbiont strains. Some vent mussels hedge their bets by maintaining both sulfur-oxidizing and methane-oxidizing bacteria, ensuring they can exploit whatever chemicals are most abundant at their particular location.
Tubeworm larvae undergo a dramatic metamorphosis: once they ingest the right bacteria, their primitive gut completely dissolves and transforms into the trophosome, permanently sealing them into symbiosis.
Living with billions of bacteria inside your body sounds like an infection waiting to happen. Yet these symbioses remain remarkably stable over the host's lifetime, sometimes decades. How do these animals manage what amounts to a controlled infection?
Recent genomic research reveals that the bacterial symbionts themselves carry sophisticated defense systems, including CRISPR-Cas arrays—the same molecular machinery that's revolutionizing genetic engineering in labs. These systems protect the bacteria from viral infections that could destabilize the symbiosis. Meanwhile, the host carefully regulates oxygen and sulfide delivery, creating chemical conditions that favor their beneficial bacteria while inhibiting potential competitors or pathogens.

The trophosome represents a massive metabolic investment—40 to 50% of a tubeworm's total body weight. To put that in perspective, your brain uses about 20% of your energy despite being only 2% of your body weight, and we think that's expensive. Tubeworms dedicate half their mass to bacterial cultivation. This investment only makes sense because the payoff is enormous: tubeworms can grow up to 1.5 meters in less than two years, making them some of the fastest-growing marine invertebrates known.
The bacteria, for their part, have streamlined genomes packed with genes for sulfur oxidation and carbon fixation while shedding many genes for independent survival. They've become so specialized for their symbiotic lifestyle that they likely couldn't survive outside a host. It's a mutual dependency that's been refined over millions of years.
The environments where these animals live would kill most organisms in minutes. Hydrothermal vent fluids can reach temperatures above 400°C and pressures exceeding 300 times atmospheric pressure—conditions so extreme that water enters a supercritical state where it's neither quite liquid nor gas. The fluids are loaded with heavy metals, acidic compounds, and concentrations of hydrogen sulfide that would be lethal to surface life.
Yet vent animals thrive in the mixing zones where superheated, chemical-rich vent water meets the frigid 2°C ocean water. They've evolved to tolerate wild temperature gradients: one side of a tubeworm might be bathed in 30°C water while the other side experiences near-freezing temperatures. Some species, like the Pompeii worm Alvinella pompejana, can tolerate tissue temperatures up to 80°C—hotter than a cup of coffee.
"These tube worm hemoglobins are remarkable for carrying oxygen in the presence of sulfide, without being inhibited by this molecule."
— Wikipedia: Riftia
These extremophile adaptations extend to the molecular level. Vent microbes carry extra copies of DNA repair genes to fix damage from heat and toxic chemicals. Host animals produce specialized proteins that protect cells from thermal stress and oxidative damage. The tubeworm's chitinous tube, which it secretes and lives inside for protection, can be remodeled at both ends through chitinolytic enzymes, allowing the worm to adjust its position to track shifting vent flows as the seafloor changes.
Hydrothermal vents aren't permanent. Volcanic activity waxes and wanes over years or decades, and vent fields can shut down abruptly when magma chambers cool or shift. When that happens, the animals face a crisis: their chemical energy source vanishes.
Research shows that tubeworm-dominated communities gradually give way to mussel and clam beds as vent activity declines. Tubeworms are colonization specialists—they grow fast and dominate early when hydrogen sulfide is abundant. But when flow decreases, slower-growing bivalves that can also filter-feed on organic particles gain an advantage. This ecological succession mirrors what happens in disturbed forests or grasslands, except here the disturbance is chemical rather than physical, and the pioneers are animals hosting bacteria rather than plants.
The ephemeral nature of vents creates a patchwork landscape where larvae must disperse potentially hundreds of kilometers to find new sites. This explains why many vent species produce enormous numbers of larvae: it's a high-stakes lottery where only a tiny fraction will stumble upon a new vent before their limited energy reserves run out.
Chemosynthesis isn't just a weird curiosity from the deep sea. Many scientists believe it may have been the first form of metabolism that evolved on Earth—predating photosynthesis by perhaps a billion years. Early Earth had abundant volcanic activity and chemical-rich oceans but relatively little oxygen. Chemosynthetic bacteria wouldn't have needed sunlight, oxygen, or complex organic molecules. Just water, carbon dioxide, and an energy-rich chemical like hydrogen sulfide or ferrous iron.
This history makes chemosynthesis particularly interesting for astrobiology. If life could bootstrap itself through chemistry on early Earth, similar processes might occur on other worlds. Icy moons like Europa and Enceladus probably have subsurface oceans in contact with rocky cores, potentially generating the same kinds of chemical gradients that fuel Earth's vent ecosystems. These moons receive barely any sunlight, but that wouldn't matter to chemosynthetic life.
The discovery of bacteria living in oceanic crust itself, deep below even the sediment layers, producing methane by combining hydrogen and carbon dioxide, hints at a vast subsurface biosphere powered entirely by geochemistry. If chemosynthetic communities can thrive in Earth's crust, similar ecosystems might exist in the porous rocks of Mars or in the ice shells of ocean worlds.
If chemosynthetic life thrives in Earth's subsurface crust and oceanic vents, similar ecosystems might exist in the icy oceans of Europa and Enceladus, or beneath the surface of Mars—no sunlight required.
What began as a shocking discovery in 1977 has blossomed into a new understanding of how adaptable life can be. Chemosynthetic symbioses demonstrate that evolution can jury-rig solutions we'd never imagine: animals that eat by not eating, that survive by housing what amounts to a controlled infection, that thrive in conditions that would sterilize a surgical instrument.
These partnerships have independently evolved at least a dozen times across the animal kingdom. They've enabled colonization of some of Earth's most chemically hostile environments. And they've shown that the fundamental requirements for complex ecosystems—energy flow, nutrient cycling, predator-prey interactions—don't necessarily need sunlight at all.
The giant tubeworm, mouth-less and magnificent, stands as evolution's proof that life doesn't follow the rules we think it should. It writes its own rules, and they're stranger and more elegant than we ever expected.
Every year, deep-sea expeditions discover new vent fields, new species, and new variations on the chemosynthetic theme. Some host combinations of sulfur and methane bacteria. Others cultivate symbionts on their backs or in their mouthparts. A few appear to farm bacteria externally, like underwater gardeners tending invisible crops.
We're still mapping this hidden world, still learning its biochemical secrets, still being surprised by evolution's creativity in the planet's darkest corners. Because it turns out that when you remove every advantage we take for granted—sunlight, warmth, low pressure, abundant food—life doesn't give up. It finds another way. It partners with bacteria that eat poison and turns the ocean's most forbidding zones into thriving cities of the strange.
The animals that gave up eating might be the most sophisticated eaters of all.
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.