Epigenetic Clocks Predict Disease 30 Years Early

TL;DR: Photobiomodulation uses specific wavelengths of red and near-infrared light (630-850 nm) to stimulate cellular repair, reduce inflammation, and boost energy production by up to 200% through mitochondrial activation. With FDA clearances for pain relief, skin rejuvenation, and vision improvement in macular degeneration, plus evidence showing 70% pain reduction in osteoarthritis and 39% increase in hair density, the therapy has crossed from experimental to established. Success depends on choosing devices with precise wavelength output (630-670 nm for skin, 810-850 nm for deep tissue), appropriate power density (10-60 mW/cm²), and following evidence-based protocols delivering 4-20 J/cm² per session, 3-5 times weekly for 8-12 weeks. The technology works through well-understood mechanisms—photons activate cytochrome c oxidase in mitochondria, triggering ATP production increases, anti-inflammatory cascades, and tissue repair pathways—making it a low-risk adjunctive therapy for conditions from chronic pain to cellular aging.

By 2030, photobiomodulation devices will be as common in households as fitness trackers—a $2.4 billion global market built on a technology NASA discovered while trying to keep astronauts healthy in space. What started as a way to heal wounds in zero gravity has evolved into a cellular-level therapy that's rewriting our understanding of how light interacts with human biology. The red glow emanating from clinics, gyms, and bedrooms worldwide isn't just ambient lighting; it's delivering specific wavelengths that penetrate your skin, enter your cells' mitochondria, and trigger a cascade of healing processes that scientists are only beginning to fully map.
The promise is extraordinary: pain relief without pills, skin rejuvenation without surgery, energy boosts without stimulants, and tissue repair accelerated by your body's own mechanisms. But as with any breakthrough that sounds too good to be true, the critical question isn't whether photobiomodulation works—rigorous studies confirm it does—but rather how to separate evidence-based applications from marketing hype, clinical-grade devices from expensive placebo lamps, and realistic expectations from miracle claims.
In the late 1960s, Hungarian physician Endre Mester stumbled upon photobiomodulation while attempting to replicate experiments using lasers to destroy tumors in rats. He miscalculated the power settings, delivering far weaker light than intended. The tumors didn't shrink, but something unexpected happened: hair grew back faster on the shaved areas exposed to the low-level laser light. This serendipitous observation launched decades of research into what scientists now call photobiomodulation (PBM)—the use of red and near-infrared light (typically 630-850 nanometers) to stimulate cellular repair, reduce inflammation, and boost energy production.
Fast-forward to the 1990s, and NASA researchers were grappling with a problem unique to space travel: wounds heal poorly in microgravity, and astronauts needed a non-pharmaceutical intervention. Their experiments with LED arrays emitting 670 nm red light produced dramatic results—wounds closed significantly faster, muscle atrophy decreased, and cellular energy production spiked by 150-200% in some trials. This wasn't pseudoscience or placebo effect; it was reproducible biochemistry happening inside mitochondria, the energy factories of every cell.
The mechanism centers on cytochrome c oxidase, a key enzyme in your mitochondrial respiratory chain. When red or near-infrared photons strike this enzyme, they displace nitric oxide molecules that normally inhibit energy production. Free from this brake, your mitochondria ramp up synthesis of adenosine triphosphate (ATP)—the molecular currency that powers every biological process from muscle contraction to neurotransmitter production. Studies measuring ATP levels in treated tissue consistently show 19-40% increases depending on wavelength, dose, and tissue type. This energy surge doesn't just fuel existing processes; it activates dormant repair pathways, shifts immune cells from inflammatory to healing states, and triggers gene expression changes that promote cell survival.
The journey from NASA laboratories to consumer devices mirrors the arc of many medical technologies—from specialized clinical tools to mass-market wellness products. But unlike fitness trackers or meditation apps, photobiomodulation devices deliver measurable biophysical effects. The light isn't merely relaxing or motivational; photons are absorbed by specific molecules in predictable ways, producing dose-dependent cellular responses.
The first commercial applications targeted pain management. In 2002, the FDA cleared low-level laser therapy devices for temporary relief of minor muscle and joint pain. This regulatory milestone validated what physical therapists and sports medicine clinics had observed empirically: patients treated with specific wavelengths of red and near-infrared light reported significant pain reduction, often comparable to pharmaceutical interventions but without side effects. A 2018 meta-analysis of 13 randomized controlled trials found that photobiomodulation reduced pain scores by an average of 70% in patients with knee osteoarthritis—a chronic condition notoriously resistant to treatment.
Skin rejuvenation followed closely behind. Dermatologists discovered that red light at 630-660 nm stimulates fibroblasts to produce more collagen and elastin while reducing matrix metalloproteinases—enzymes that degrade skin structure. In one study, over 90% of participants reported smoother skin, reduced redness, and lightening of dark spots after just eight LED sessions over four weeks. The FDA has since cleared multiple devices for wrinkle reduction, acne treatment, and skin texture improvement. What distinguishes these approved devices from cosmetic gimmicks is precisely calibrated wavelength output, power density, and treatment protocols derived from controlled trials.
Hair restoration represents perhaps the most consumer-visible success story. Low-level laser therapy (LLLT) for androgenetic alopecia (pattern baldness) earned FDA clearance after randomized studies demonstrated a 39% increase in hair density over 16 weeks in male participants. The mechanism involves stimulating hair follicle stem cells with 680 nm light—the optimal wavelength for reaching the follicle bulb beneath the scalp's dense outer layer. Critically, coherent laser light proved far more effective than LED arrays because lasers deliver focused, single-wavelength beams with sufficient power density to penetrate to target depth. Simply adding more LEDs cannot compensate for their inherent lack of coherence and lower intensity.
Understanding photobiomodulation requires zooming into the molecular ballet happening inside your cells when red or near-infrared photons arrive. Think of it as a precisely tuned key unlocking specific biochemical doors—not through heat or ionization, but through selective absorption by photoreceptor molecules.
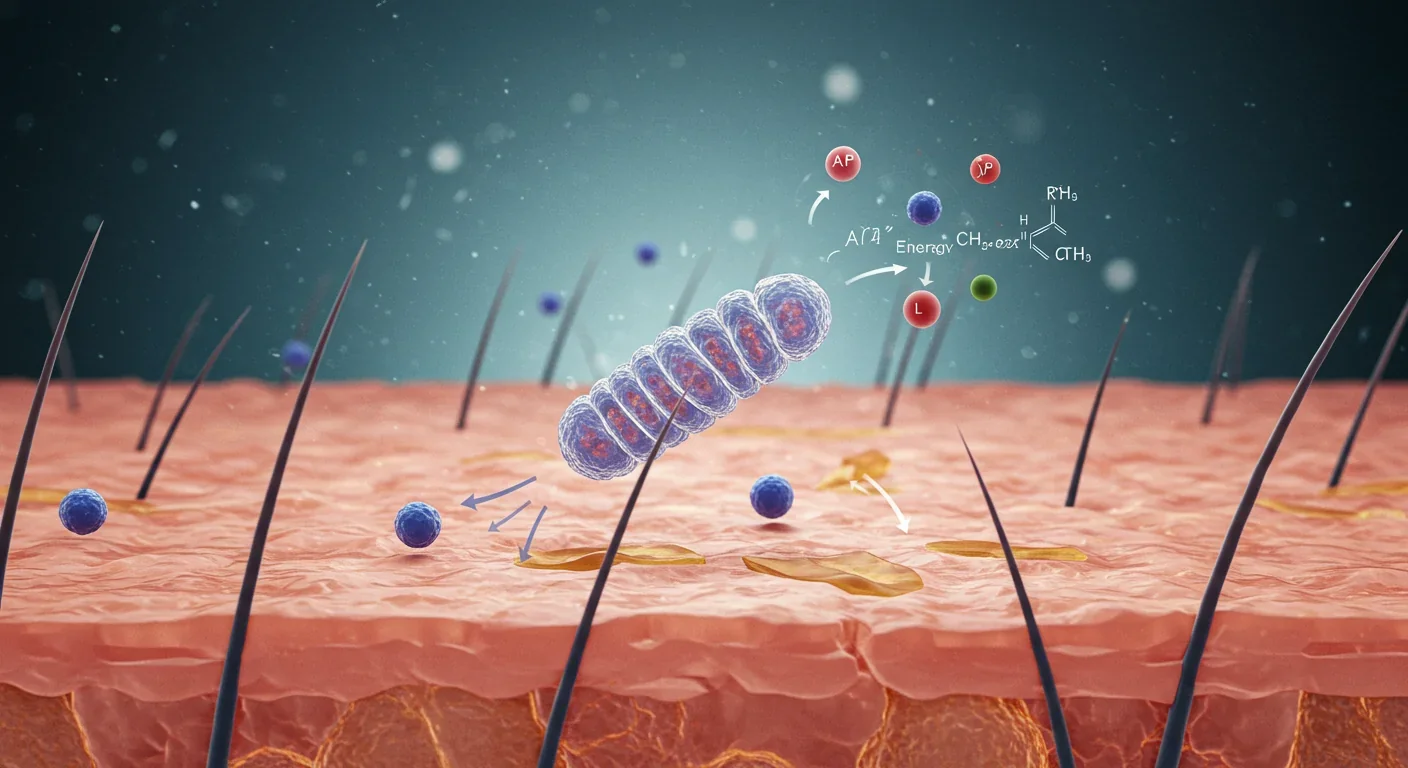
The primary photoreceptor is cytochrome c oxidase (CcO), the terminal enzyme in your mitochondrial electron transport chain. Under normal conditions, nitric oxide (NO) binds to CcO's active site, partially inhibiting its activity—a natural brake on energy production. When photons in the 600-850 nm range strike CcO, they photodissociate this nitric oxide, removing the inhibition. Freed from this constraint, CcO accelerates electron transfer, pumps more protons across the mitochondrial membrane, and drives ATP synthase to produce more ATP. Researchers measuring this effect in real time observe oxygen consumption dropping initially as the light inhibits CcO, then surging as the enzyme rebounds to control levels within 2.5 hours—a therapeutic window that explains optimal treatment timing.
But energy production is only the beginning. The transient increase in reactive oxygen species (ROS) that accompanies photobiomodulation acts as a signaling mechanism, not cellular damage. At low to moderate doses (4-20 J/cm²), this controlled oxidative burst activates transcription factors like NF-κB and AP-1, which migrate to the nucleus and upregulate genes encoding antioxidant enzymes, anti-inflammatory cytokines, and growth factors. This explains the seemingly paradoxical finding that light therapy both increases ROS initially and reduces oxidative stress long-term—it's triggering your cells' endogenous protective systems.
The inflammatory modulation is particularly striking. Studies across multiple tissue types show photobiomodulation downregulates pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) while upregulating anti-inflammatory IL-10. In macrophages—immune cells central to inflammation—PBM shifts metabolism from glycolysis (which fuels the pro-inflammatory M1 phenotype) to oxidative phosphorylation (which characterizes anti-inflammatory M2 macrophages). This metabolic reprogramming explains why PBM reduces swelling, pain, and tissue destruction in conditions from arthritis to retinal degeneration.
Neuroprotection adds another dimension. In models of stroke and traumatic brain injury, applying near-infrared light during the early reperfusion period prevents mitochondrial hyperpolarization—an electrical imbalance that triggers cell death cascades. By partially inhibiting cytochrome oxidase at the moment blood flow resumes, the light prevents the massive ROS burst that would otherwise destroy neurons. Within hours, enzyme activity returns to normal, but the cells have avoided catastrophic damage. This precise timing—intervention during a critical therapeutic window—illustrates why photobiomodulation protocols must be evidence-based, not guesswork.
Photobiomodulation's credibility rests on a foundation of peer-reviewed studies, randomized controlled trials, and FDA clearances that separate it from wellness fads. The evidence is strongest for pain management, wound healing, and tissue repair—applications where mechanistic understanding aligns with clinical outcomes.
For osteoarthritis, the data is compelling. Animal models using monoiodoacetate-induced joint degeneration show that near-infrared laser at 57 J/cm² produces greater pain relief and improved range of motion than higher doses—illustrating the biphasic dose response characteristic of photobiomodulation. Too little light fails to trigger therapeutic cascades; too much can inhibit them. In rats treated with 810 nm light at 18 J/cm², inflammatory cytokines dropped significantly while antioxidant enzymes increased. Human trials mirror these findings: a systematic review concluded that consistent PBM application measurably reduces pain and improves function in knee osteoarthritis patients, with effect sizes comparable to pharmaceutical interventions.
Wound healing demonstrates photobiomodulation at its most dramatic. In diabetic rats—whose impaired healing mimics a major clinical challenge—low-level laser therapy (630-660 nm, 6 J/cm²) accelerated closure to 100% by day 10, matching healthy control rates. The treated wounds showed optimal healing at day 7, during the transition from inflammation to proliferation, suggesting timing matters as much as dose. Mechanistically, the light upregulates tissue inhibitors of metalloproteinases (TIMPs) while downregulating matrix metalloproteinases (MMPs)—shifting the balance from tissue breakdown to reconstruction. In oral mucosal ulcers, laser-treated groups achieved significantly higher healing percentages with concurrent improvements in oxidative stress markers.
Retinal disease represents a frontier where photobiomodulation is earning regulatory acceptance. In November 2024, the FDA authorized Valeda, a photobiomodulation device delivering red (590 nm), yellow (660 nm), and near-infrared (850 nm) light for dry age-related macular degeneration (AMD). The LIGHTSITE II trial showed that 35.3% of eyes receiving all 27 treatments gained five or more letters of visual acuity, with 20% less geographic atrophy progression compared to sham treatment. The LIGHTSITE III study extended these findings over 13 months, demonstrating sustained benefit and lower incidence of new atrophy. The treatment targets mitochondrial dysfunction in retinal cells—tissues exceptionally rich in mitochondria and vulnerable to oxidative stress. By enhancing cytochrome oxidase activity and shifting macrophages to anti-inflammatory phenotypes, photobiomodulation slows retinal degeneration through mechanisms entirely distinct from drug therapy.
For myopia prevention in children, the evidence is emerging but striking. A randomized controlled trial of 188 children using twice-daily photobiomodulation showed significant reduction in axial eye elongation—the structural change driving progressive nearsightedness. The mechanism likely involves mitochondrial stimulation in retinal cells and enhanced dopamine signaling, offering a non-pharmaceutical prophylactic strategy for populations at high myopia risk.
Not every application has equal evidence. Claims about weight loss, for instance, rest on more preliminary data. Studies do show that red light therapy can create temporary openings in adipocyte membranes, releasing fatty acids into circulation—but without concurrent exercise, these lipids simply get re-stored. A 2011 trial found significant waist circumference reduction when participants used red light on the abdomen twice weekly for four weeks, but effect sizes are modest (2-5 cm) and require sustained lifestyle modification to maintain.
The photobiomodulation device market has exploded from clinical-grade lasers costing tens of thousands to consumer LED panels priced under $200. This democratization brings both opportunity and confusion—how do you distinguish therapeutic devices from expensive mood lighting?
Wavelength precision is non-negotiable. Effective photobiomodulation requires specific wavelengths: red light at 630-670 nm for surface tissue effects (skin, superficial wounds) and near-infrared at 810-850 nm for deeper penetration (muscle, joints, brain tissue). Some advanced devices add 1060 nm for maximum depth. Quality manufacturers specify exact wavelengths with narrow bands (±5 nm); if a product lists vague ranges or "red light therapy" without nanometer specifications, it's a red flag. The Swedish Laser Medical Society identifies 680 nm as optimal for hair follicle stimulation, while retinal applications cluster around 670, 590, and 850 nm—precision matters because different wavelengths activate different chromophores.
Power density (irradiance) determines treatment time and efficacy. Measured in milliwatts per square centimeter (mW/cm²), this metric indicates how much light energy reaches your tissue per unit time. Clinical studies typically use 10-60 mW/cm² for most applications. Home panels range from 30-100 mW/cm², while handheld devices may deliver 5-30 mW/cm². Higher irradiance isn't necessarily better—remember the biphasic dose response—but it allows shorter treatment sessions to reach therapeutic dose (measured in joules per square centimeter, J/cm²). For example, a 50 mW/cm² device delivers 10 J/cm² in about 3.3 minutes, while a 20 mW/cm² device needs 8.3 minutes for the same dose. Devices that don't specify power density make proper dosing impossible.
Total dose (fluence) is where treatment protocols succeed or fail. The cumulative energy delivered per session—calculated as irradiance × time—must fall within evidence-based therapeutic windows. For pain relief, studies cluster around 4-20 J/cm². Skin rejuvenation uses similar ranges, while wound healing may go higher. The key is avoiding the trap of "more is better": excessive doses (>50 J/cm² in many contexts) can inhibit the very processes you're trying to stimulate. Quality devices include timers or automatic shutoff to prevent overdosing.
LED vs. laser is context-dependent. For hair growth, lasers win decisively because coherence and single-wavelength focus enable penetration to follicle stem cells; LEDs simply can't reach that depth regardless of quantity. Theradome's claim that lasers are "60,000 times stronger than LEDs" is marketing hyperbole, but the principle is sound: for deep-tissue targets, laser coherence matters. For surface applications like facial skin treatment, high-quality LED panels work excellently at lower cost and with broader treatment areas. FDA-cleared LED masks for wrinkle reduction demonstrate clinical efficacy because the target (dermal fibroblasts) sits only 1-2 mm below the skin surface.
Device design affects real-world usability. Full-body panels offer maximum coverage but require dedicated space and 10-20 minute sessions. Targeted panels (face, joints) provide portability and convenience, often with integrated timers. Eye-specific devices like heated wands deliver precision for periorbital treatment without risk of inadvertent upper-eyelid exposure—important because direct retinal irradiation from general-purpose panels can carry risk, especially for people with glaucoma or retinal disease. Flicker-free technology reduces headache risk from rapid light fluctuations. Adjustable intensity allows gradual acclimation, starting at 50-60% of maximum to avoid discomfort.
Photobiomodulation's safety profile is remarkably benign compared to pharmaceutical and surgical alternatives—but "generally safe" doesn't mean "safe for everyone" or "impossible to misuse."
The most common side effects are transient and mild: temporary skin redness or warmth during treatment, occasional headaches from light flicker (mitigated by flicker-free devices), and initial dizziness or irritability in the first few days of bright light therapy (this typically resolves as circadian rhythms adjust). Clinical trials for ocular photobiomodulation report temporary increases in dry eye symptoms but no serious adverse events. Studies testing doses up to 320 J/cm² for darker skin tones and 480 J/cm² for other skin types found no significant adverse effects—far above typical therapeutic doses.
Eye exposure requires specific precautions. General-purpose red light panels should not be used with eyes open at close range; even though red and near-infrared light lack the DNA-damaging capacity of ultraviolet, high-intensity exposure can cause retinal stress or exacerbate conditions like macular degeneration if applied incorrectly. Eye-targeted devices (like Valeda or specialized periorbital wands) use precisely calibrated intensities, wavelengths, and exposure times developed for ocular safety. If using a facial mask or panel, either close your eyes or use provided goggles. Never use devices not designed for eyes directly on the upper eyelid or look directly into high-power LEDs.

Photosensitivity medications create contraindications. Antibiotics like tetracyclines, certain antihypertensives, and some psychiatric medications increase skin sensitivity to light. While photobiomodulation doesn't use ultraviolet wavelengths, the added energy load could trigger exaggerated inflammatory responses in photosensitized tissue. Always disclose medications to practitioners and consult your physician before starting home treatment if taking photosensitizing drugs.
Active cancer is a theoretical concern requiring caution. While no evidence suggests photobiomodulation promotes tumor growth, and some studies explore its use to mitigate radiation damage in cancer patients, the theoretical possibility that enhanced cellular energy could support malignant cells leads most protocols to list active cancer as a contraindication. If you're in remission or cleared by your oncologist, the risk calculus changes—but this requires medical consultation, not self-determination.
Other contraindications include pregnancy (insufficient safety data), open wounds or active infections in the treatment area, fever or acute illness, hyperthyroidism (if treating thyroid region), and epilepsy triggered by light. Timing matters for circadian effects: bright red light exposure within 2-3 hours of bedtime can suppress melatonin and delay sleep onset. Dose discipline prevents the biphasic trap—stick to evidence-based protocols of 10-20 minutes per body area, 3-5 times weekly, at validated fluences of 4-20 J/cm².
The gap between clinical trial outcomes and home-use results often comes down to protocol adherence, device quality, and appropriate condition selection. Understanding what photobiomodulation can realistically achieve—and how long it takes—sets the foundation for success rather than disappointment.
Athletic recovery: Professional and collegiate sports teams now routinely use photobiomodulation for delayed-onset muscle soreness and injury recovery. A study in the Journal of Strength and Conditioning Research found that athletes receiving red light therapy had 40% less muscle soreness and regained original strength 24-48 hours faster than controls. The practical application: using a near-infrared panel on major muscle groups for 10-15 minutes post-workout, 3-5 times weekly. Results appear within days for soreness reduction, weeks for cumulative performance benefits. The key is pairing photobiomodulation with proper recovery fundamentals—sleep, nutrition, hydration—not replacing them.
Chronic pain management: A comprehensive review of 11 clinical studies confirmed significant pain relief and improved function in arthritis patients using photobiomodulation. Real-world protocols typically involve 8-12 week treatment courses, 3 times weekly, with some patients experiencing 30-50% pain reduction. The timeline is gradual—initial improvement around week 2-3, plateau around week 8-10. This isn't a miracle cure eliminating all pain overnight; it's a tool reducing inflammation and enhancing tissue repair that, for many, provides meaningful relief without pharmaceutical side effects. Combining photobiomodulation with physical therapy often produces superior results to either alone.
Skin aging and rejuvenation: Users of LED facial devices report visible improvements in skin texture within 2-4 weeks, with cumulative benefits over 8-12 weeks. One patient testimonial described a 30-year-old keloid scar smoothing to skin surface level and returning to original skin tone after consistent treatment—a dramatic outcome illustrating photobiomodulation's collagen-remodeling capacity. More typical results include subtle firming, reduced fine lines, more even tone, and improved product absorption when combining red light with topical serums. The caveat: results depend on baseline skin condition, age, and lifestyle factors (sun protection, diet, smoking). Photobiomodulation enhances your skin's regenerative capacity; it doesn't override chronic damage.
Mood and cognitive function: Emerging research suggests transcranial photobiomodulation improves depressive symptoms and cognitive flexibility. A 2019 randomized trial found significant improvement in treatment-resistant depression after eight weeks of therapy. The mechanism—enhanced mitochondrial ATP production, improved cerebral blood flow, and synaptic plasticity modulation—makes neurological applications scientifically plausible. Combining photobiomodulation with pulsed electromagnetic field (PEMF) therapy or quantitative EEG-guided transcranial magnetic stimulation (TMS) produces synergistic effects, leading to faster symptom relief and longer-lasting improvement.
Hair restoration: Users of FDA-cleared laser devices report gradual improvements in hair density over 16-26 weeks—not overnight regrowth, but measurable increases in terminal hair count and diameter. The key is early intervention (treating thinning before follicles die completely) and consistency (3-5 sessions weekly without interruption). LED devices marketed for hair growth generally underperform because they lack the coherence and power density to reach follicle stem cells.
Photobiomodulation research is expanding beyond established applications into domains that could reshape clinical practice over the next decade. Metabolic disorders represent an emerging target—early data suggests red light therapy might influence adipocyte function beyond simple fat release, potentially targeting metabolic dysregulation in type 2 diabetes and non-alcoholic fatty liver disease. By enhancing mitochondrial function in insulin-sensitive tissues, photobiomodulation could improve glucose metabolism independent of weight loss.
Senolytic therapy combinations offer another frontier. Cellular senescence—when cells stop dividing but don't die, instead secreting inflammatory molecules—drives vascular aging and chronic disease. Energy-based devices, particularly when combined with topical antioxidants, can downregulate senescence-associated secretory phenotype (SASP) genes more effectively than either alone. Applying similar combinatorial approaches to red light therapy could amplify anti-inflammatory and rejuvenation effects.
Neuroprotection and regeneration show remarkable promise. The discovery that near-infrared light applied during early reperfusion after stroke prevents mitochondrial catastrophe opens possibilities for emergency medical services to administer photobiomodulation in the field or trauma bay. Portable transcranial devices could provide immediate neuroprotection while patients are transported to comprehensive stroke centers.
Corporate and regulatory momentum is accelerating. Alcon's acquisition of LumiThera and the Valeda device signals that major ophthalmology companies see photobiomodulation as a viable treatment modality, not a fringe therapy. FDA authorization for dry AMD demonstrates regulatory confidence in safety and efficacy. This corporate validation could accelerate photobiomodulation's adoption across other medical specialties where mitochondrial dysfunction plays a central role: Parkinson's disease, Alzheimer's disease, chronic fatigue syndrome.
The future likely involves photobiomodulation not as monotherapy but as part of multimodal treatment. Combining PBM with PEMF enhances both mitochondrial energy production and cellular signaling, producing faster and more sustained outcomes. Pairing photobiomodulation with stem cell therapy in osteoarthritis synergistically downregulates degradative enzymes and upregulates protective factors, offering regenerative potential beyond single-modality approaches. Integrating photobiomodulation with quantitative EEG-guided TMS creates neuroplastic effects stronger and longer-lasting than either alone.
Translating evidence into personal benefit requires matching your goals to appropriate protocols and devices. For pain relief from arthritis, back pain, or sports injuries, use 810-850 nm near-infrared wavelengths with a handheld panel or targeted pad. Apply 30-60 mW/cm² power density for 10-15 minutes per area, 3-5 times weekly, delivering 10-20 J/cm² per treatment. Position the device 6-12 inches from your skin. Expect initial improvement at 2-3 weeks, plateau at 8-10 weeks, then maintain with 2-3 sessions weekly.
For skin rejuvenation and anti-aging, choose 630-670 nm red light using an LED face mask or panel designed for facial contours. Apply 20-50 mW/cm² for 10-20 minutes, 4-5 times weekly, delivering 10-15 J/cm². Apply antioxidant serum before treatment for synergistic effects. Expect texture improvement at 2-4 weeks, cumulative firming and tone evening over 8-12 weeks.
For athletic recovery and performance, use 810-850 nm NIR or combination 660nm + 850nm on major muscle groups with a large panel. Apply 50-100 mW/cm² for 10-15 minutes post-workout, delivering 15-20 J/cm². Treat immediately or within 3 hours of exercise. Expect reduced soreness within 24-48 hours and faster strength recovery, with cumulative adaptation over weeks.
For mood and energy, use bright white light (10,000 lux) for seasonal affective disorder or red/NIR combination for mitochondrial stimulation. Apply light therapy box (10,000 lux, 200+ square inches) for 30 minutes each morning within 1 hour of waking for SAD. Use transcranial NIR device for 10-15 minutes, 3-5 times weekly for mitochondrial support. Schedule morning only—avoid within 3 hours of bedtime. Expect mood improvement at 1-2 weeks for SAD with sustained daily use.
For hair restoration, use 680 nm laser (not LED) with an FDA-cleared laser cap or helmet. Verify the device uses laser diodes, not LEDs. Apply for 20-30 minutes, 3-5 times weekly, minimum 16-week initial course. Expect visible improvement at 12-16 weeks if starting with thinning (not bald) areas, then maintain indefinitely to sustain gains.
Always start intensity at 50-60% of device maximum and increase gradually. Use eye protection or close your eyes unless the device is specifically eye-safe. Avoid direct treatment over tattoos. Discontinue if skin becomes hot, painful, or develops rash. Document treatment times to prevent accidental overdosing. Maintain your device per manufacturer specifications.
Photobiomodulation represents a profound shift in how we think about therapeutic interventions—not adding a chemical to alter biochemistry, but delivering energy in a form your cells evolved to use. The red and near-infrared wavelengths penetrating your skin aren't foreign substances requiring metabolism and clearance; they're photons that specific molecules absorb to perform work, the same fundamental process that powers photosynthesis in plants.
The evidence base is now substantial: thousands of peer-reviewed studies, dozens of randomized controlled trials, FDA clearances for multiple indications, and adoption by major healthcare systems and professional sports organizations. This isn't fringe medicine or wellness theater; it's biophysics applied to clinical problems. When a Stanford study shows 70% pain reduction in knee osteoarthritis, when NASA documents 150% increases in cellular ATP, when the FDA authorizes a device for vision improvement in macular degeneration—photobiomodulation has crossed the threshold from experimental to established.
Yet effectiveness depends entirely on proper implementation. The $79 LED bulb screwed into a desk lamp delivers red light, but not at the wavelength precision, power density, or dose control that clinical studies used. The $4,000 full-body panel with nine wavelengths provides those specs but may be overkill if you're targeting facial skin alone. Success requires matching device capabilities to evidence-based protocols for your specific condition, then executing those protocols with the same consistency you'd apply to physical therapy exercises or medication schedules.
The realistic promise: photobiomodulation won't reverse decades of joint degeneration, eliminate deep wrinkles overnight, or regrow a full head of hair from completely bald follicles. But it can meaningfully reduce pain and inflammation, stimulate measurable improvements in skin structure and appearance, and slow or partially reverse hair thinning—outcomes that improve quality of life without pharmaceutical side effects or surgical risks. For athletes, it offers faster recovery and reduced soreness. For aging populations, it provides a tool to maintain cellular energy production as mitochondrial function naturally declines. For patients with conditions like dry AMD or chronic pain, it opens treatment possibilities where few existed.
The future will likely see photobiomodulation integrated into multimodal protocols—combined with stem cells for joint regeneration, paired with TMS for neuropsychiatric conditions, used alongside cancer treatment to mitigate radiation damage, incorporated into post-surgical recovery to accelerate wound healing. As precision medicine advances, protocols will become individualized based on genetic variants affecting mitochondrial function, tissue-specific dosing guided by real-time sensors, and combination wavelengths tailored to your unique cellular chromophore profile.
For now, the actionable insight is clear: photobiomodulation works through well-understood mechanisms, produces measurable effects in properly designed studies, and offers a low-risk adjunctive therapy for numerous conditions—provided you choose quality devices, follow evidence-based protocols, and maintain realistic expectations. The red glow emanating from clinics and living rooms worldwide isn't magic; it's molecular biology harnessed for healing. Your cells know what to do with that light; the question is whether you'll give them the wavelengths, dose, and consistency they need to do it.

Recent breakthroughs in fusion technology—including 351,000-gauss magnetic fields, AI-driven plasma diagnostics, and net energy gain at the National Ignition Facility—are transforming fusion propulsion from science fiction to engineering frontier. Scientists now have a realistic pathway to accelerate spacecraft to 10% of light speed, enabling a 43-year journey to Alpha Centauri. While challenges remain in miniaturization, neutron management, and sustained operation, the physics barriers have ...

Epigenetic clocks measure DNA methylation patterns to calculate biological age, which predicts disease risk up to 30 years before symptoms appear. Landmark studies show that accelerated epigenetic aging forecasts cardiovascular disease, diabetes, and neurodegeneration with remarkable accuracy. Lifestyle interventions—Mediterranean diet, structured exercise, quality sleep, stress management—can measurably reverse biological aging, reducing epigenetic age by 1-2 years within months. Commercial ...

Data centers consumed 415 terawatt-hours of electricity in 2024 and will nearly double that by 2030, driven by AI's insatiable energy appetite. Despite tech giants' renewable pledges, actual emissions are up to 662% higher than reported due to accounting loopholes. A digital pollution tax—similar to Europe's carbon border tariff—could finally force the industry to invest in efficiency technologies like liquid cooling, waste heat recovery, and time-matched renewable power, transforming volunta...

Humans are hardwired to see invisible agents—gods, ghosts, conspiracies—thanks to the Hyperactive Agency Detection Device (HADD), an evolutionary survival mechanism that favored false alarms over fatal misses. This cognitive bias, rooted in brain regions like the temporoparietal junction and medial prefrontal cortex, generates religious beliefs, animistic worldviews, and conspiracy theories across all cultures. Understanding HADD doesn't eliminate belief, but it helps us recognize when our pa...

The bombardier beetle has perfected a chemical defense system that human engineers are still trying to replicate: a two-chamber micro-combustion engine that mixes hydroquinone and hydrogen peroxide to create explosive 100°C sprays at up to 500 pulses per second, aimed with 270-degree precision. This tiny insect's biochemical marvel is inspiring revolutionary technologies in aerospace propulsion, pharmaceutical delivery, and fire suppression. By 2030, beetle-inspired systems could position sat...

The U.S. faces a catastrophic care worker shortage driven by poverty-level wages, overwhelming burnout, and systemic undervaluation. With 99% of nursing homes hiring and 9.7 million openings projected by 2034, the crisis threatens patient safety, family stability, and economic productivity. Evidence-based solutions—wage reforms, streamlined training, technology integration, and policy enforcement—exist and work, but require sustained political will and cultural recognition that caregiving is ...

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.