Interoception Training: Stop Anxiety & Chronic Pain Naturally

TL;DR: Mitophagy—your cells' cleanup mechanism for damaged mitochondria—holds the key to preventing Parkinson's, Alzheimer's, heart disease, and diabetes. Scientists have discovered you can boost this process through exercise, fasting, and specific compounds like spermidine and urolithin A.
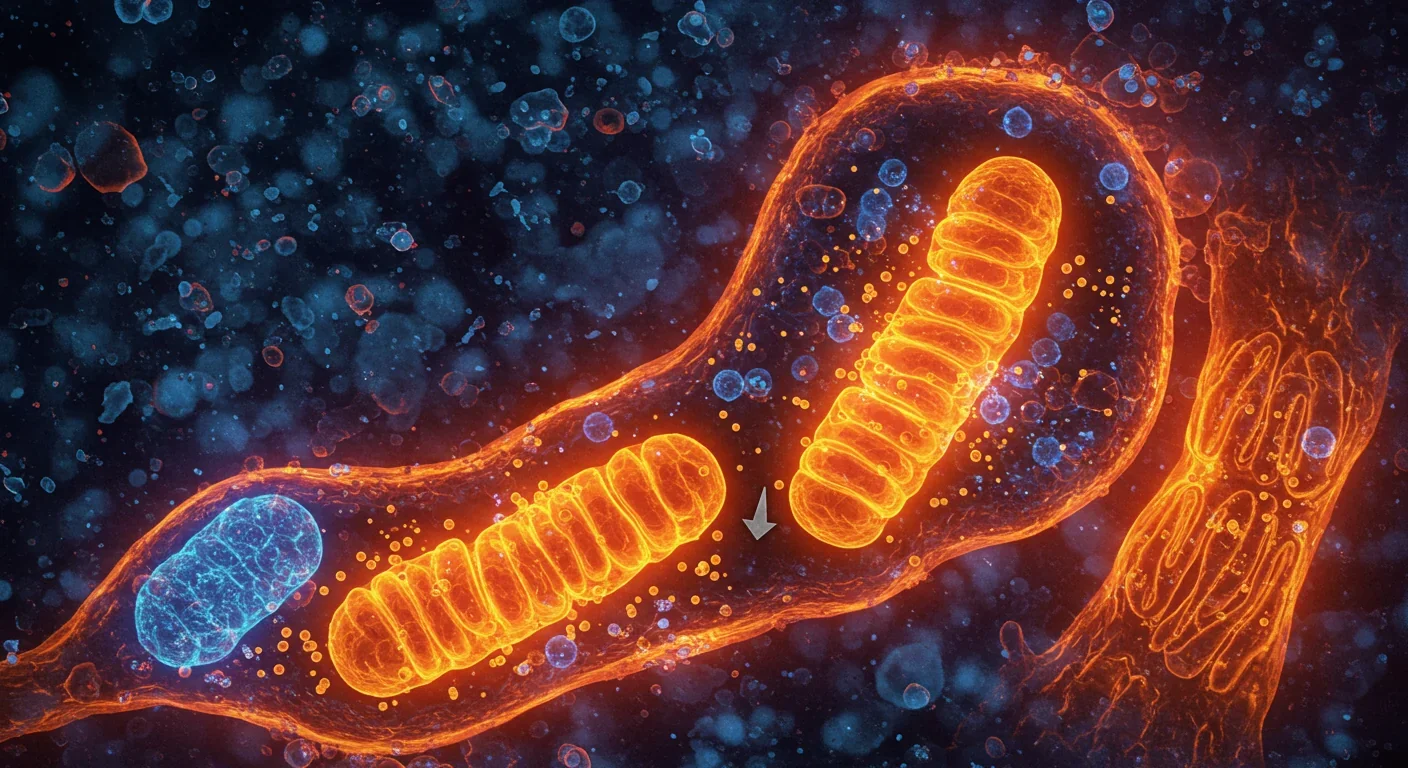
Imagine your cells conducting a nightly cleanup crew, systematically removing broken machinery before it poisons the entire system. That's mitophagy—your body's quality control mechanism for mitochondria, the powerhouses that keep every cell running. Scientists now believe that when this cleanup process slows down, we age. When it fails completely, we get Parkinson's, Alzheimer's, heart disease, and diabetes. But here's the exciting part: we're learning how to turn the cleanup crew back on.
Every cell in your body contains hundreds or thousands of mitochondria, tiny organelles that convert food into energy. Over time, these powerhouses accumulate damage from the very process of generating energy—oxidative stress creates toxic byproducts that corrode mitochondrial DNA and proteins. Left unchecked, damaged mitochondria leak inflammatory signals that accelerate cellular aging and trigger chronic diseases.
Mitophagy is the answer evolution came up with. The name combines "mitochondria" with "autophagy," the cell's general recycling system. Think of autophagy as municipal waste management, while mitophagy is the specialized hazmat team that handles toxic waste. When a mitochondrion becomes damaged beyond repair, molecular sensors tag it for destruction. The cell then wraps it in a membrane bubble and digests it, recycling the components into fresh building materials.
This isn't just theoretical housekeeping. Research shows that mitophagy dysfunction sits at the intersection of aging and major diseases. When mitophagy works efficiently, cells stay energized and inflammation stays low. When it falters, damaged mitochondria pile up like garbage during a sanitation strike, and the whole neighborhood suffers.
The connection between mitophagy and neurodegenerative disease started to crystallize about 15 years ago, when geneticists discovered that mutations in two proteins—PINK1 and Parkin—cause early-onset Parkinson's disease. These proteins turned out to be the molecular tags that mark damaged mitochondria for destruction. Without them, neurons accumulate defective mitochondria until the energy crisis becomes fatal.
The mechanism is elegant and brutal. PINK1 normally sits on the outer membrane of healthy mitochondria, where it gets constantly degraded. But when a mitochondrion loses its membrane potential—the electrical charge that indicates it's working—PINK1 accumulates on the surface like a distress beacon. This recruits Parkin, which slaps molecular tags onto the damaged organelle, calling in the autophagy machinery to consume it.
Recent breakthroughs have visualized this process with unprecedented clarity. Scientists at Harvard just revealed how PINK1 detects mitochondrial damage by monitoring the protein import machinery—essentially checking whether new proteins can successfully enter the mitochondrion. If the import gate is jammed, PINK1 sounds the alarm.
But Parkinson's is just the beginning. Mitochondrial dysfunction appears central to Alzheimer's disease, where impaired mitophagy allows damaged mitochondria to accumulate in neurons, creating oxidative stress that accelerates the buildup of toxic amyloid plaques and tau tangles. The pattern repeats across neurodegenerative conditions—ALS, Huntington's, even normal cognitive aging all show signatures of inadequate mitochondrial quality control.
While neuroscientists were focused on the brain, cardiologists discovered that mitophagy protects the heart. Cardiac muscle cells are absolutely packed with mitochondria because they need to contract billions of times without rest. When mitophagy declines, damaged mitochondria accumulate in heart tissue, contributing to heart failure with preserved ejection fraction—a condition where the heart muscle stiffens and can't relax properly between beats.
The cardiovascular connection runs deeper. Mitophagy helps regulate inflammation in blood vessel walls, protecting against atherosclerosis and coronary heart disease. When this process fails, damaged mitochondria release inflammatory signals that destabilize arterial plaques, increasing heart attack risk.
Metabolic disease tells a similar story. Type 2 diabetes involves mitochondrial dysfunction at multiple levels—pancreatic beta cells can't secrete insulin properly, muscle cells can't burn glucose efficiently, and liver cells overproduce glucose. Impaired mitophagy appears to drive this cascade. Studies show that diabetic tissues accumulate damaged mitochondria, creating oxidic stress and insulin resistance that perpetuate the disease.
The mechanistic links keep emerging. Researchers have identified how defective mitophagy contributes to obesity-related kidney damage, and how restoring mitochondrial quality control can reverse some of the metabolic dysfunction.

Here's where it gets practical. You can influence your mitophagy rates through lifestyle choices, and the evidence is surprisingly robust.
Exercise tops the list. When you work out—especially with high-intensity interval training or endurance exercise—you create mild stress that actually damages some mitochondria. This sounds counterintuitive, but the damage triggers mitophagy to clear out the weakest mitochondria while simultaneously stimulating the creation of new, more efficient ones. The two best workouts for mitochondrial health combine strength training with high-intensity intervals, forcing muscles to adapt by upgrading their energy production capacity.
Caloric restriction and fasting activate mitophagy through multiple pathways. When nutrient levels drop, cells activate a protein called AMPK (AMP-activated protein kinase), which essentially tells cells "energy is scarce, clean up your mitochondria and work more efficiently." Even intermittent fasting appears to boost mitochondrial quality control, though you don't need to starve—a 12-16 hour overnight fast several times a week may be enough to trigger the effect.
Dietary compounds are entering the spotlight. Three molecules have particularly strong research backing:
Spermidine, found in wheat germ, soybeans, aged cheese, and mushrooms, appears to directly activate autophagy and improve mitochondrial function. Clinical studies suggest it may improve cognitive function and cardiovascular health, though most people would need supplements to reach therapeutic doses.
Urolithin A comes from pomegranates, but here's the catch—your gut bacteria have to convert compounds in pomegranate juice into urolithin A, and not everyone's microbiome can do it efficiently. The molecule enhances mitophagy and has shown promise in clinical trials for muscle function and endurance. A recent study found that urolithin A helps clear toxic proteins in Alzheimer's models, possibly by improving mitochondrial cleanup.
NAD+ boosters like nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) work by replenishing NAD+, a molecule that declines with age and is essential for mitochondrial function. While NAD+ repletion shows benefits in animal models, human trials have given mixed results, and the supplements are expensive.
Understanding mitophagy helps explain why aging hits different tissues at different rates. Neurons and cardiac muscle cells rarely divide, so they need exceptional mitochondrial quality control to last decades. When mitophagy declines—as it does in virtually everyone past age 60—these long-lived cells accumulate damage faster than renewable tissues like skin or intestinal lining.
But the decline isn't inevitable or uniform. Some people maintain robust mitophagy into old age, often those who stay physically active. Elite endurance athletes in their 60s and 70s have mitochondrial profiles that resemble those of sedentary people in their 30s.
The societal implications are staggering. If we could safely boost mitophagy by just 20-30% in middle age, we might compress decades of disability into a few years at the very end of life. Instead of a slow decline starting at 65, people might remain vigorous until 85, then experience rapid decline. That's the difference between spending retirement traveling and spending it in doctors' waiting rooms.
Drug companies have noticed this potential. Multiple clinical trials are testing compounds that specifically activate mitophagy pathways. Some target the PINK1-Parkin system directly, trying to boost the tagging of damaged mitochondria. Others work upstream, activating AMPK or inhibiting mTOR—a protein that suppresses autophagy when nutrients are abundant.
The challenge is selectivity. You want to clear damaged mitochondria without triggering excessive autophagy that destroys healthy ones. You want to activate cleanup without suppressing the creation of new mitochondria. The cell has evolved elaborate controls to balance these competing needs, and pharmaceutical intervention risks disrupting that balance.
Still, the potential rewards justify the risk. If we could develop a safe, effective mitophagy enhancer, it might become the first true anti-aging drug—not by attacking a specific disease, but by maintaining the cellular quality control that prevents multiple diseases simultaneously.

We're also seeing the emergence of biomarkers that could guide personalized interventions. Researchers can now measure mitochondrial health in blood samples, assessing damage levels and clearance rates. In theory, your doctor could test your mitophagy efficiency and recommend specific interventions if it's declining prematurely.
Some forward-thinking physicians are already incorporating this thinking. They're prescribing exercise regimens designed specifically to stress mitochondria, recommending time-restricted eating, and monitoring metabolic markers that reflect mitochondrial function. It's not quite precision medicine, but it's moving beyond generic "eat less, move more" advice.
The supplement industry, predictably, has raced ahead of the evidence. You can now buy mitophagy-boosting supplements that combine spermidine, urolithin A, and NAD+ precursors, often at prices exceeding $100 per month. Do they work? The honest answer is we don't know yet—most human trials have tested these compounds individually, not in combination, and optimal doses remain unclear.
Looking ahead, mitophagy research is converging with other longevity science in unexpected ways. Senescent cells—zombie cells that refuse to die and spew inflammatory signals—also show impaired mitophagy. Clearing these cells (an approach called senolysis) might work partly by removing cells with broken cleanup machinery. The epigenetic changes associated with aging include modifications that suppress autophagy genes, suggesting that DNA methylation patterns could be reprogrammed to restore youthful mitophagy.
We might be witnessing the early stages of a fundamental shift in how medicine approaches aging. Instead of treating each age-related disease separately—statins for heart disease, insulin for diabetes, levodopa for Parkinson's—we could target the underlying cellular maintenance systems that prevent all of them.
That future isn't here yet. But the science has advanced enough that you can make informed choices today. Exercise, particularly high-intensity training, reliably boosts mitophagy. Intermittent fasting appears to help. Certain dietary compounds show promise, though supplement quality varies wildly.
The real insight, though, is philosophical. Your cells are constantly rebuilding themselves, discarding damaged parts and synthesizing fresh replacements. Aging happens when this renewal process falls behind the damage accumulation rate. Mitophagy is one of the key renewal mechanisms, and we're learning to influence it.
In other words, the fountain of youth might not be a mythical spring—it might be your own cells' cleanup crew, working overnight while you sleep, dismantling the molecular debris before it accumulates into disease. We're just learning how to keep that crew on the job a few decades longer.
The Bussard Ramjet, proposed in 1960, would scoop interstellar hydrogen with a massive magnetic field to fuel fusion engines. Recent studies reveal fatal flaws: magnetic drag may exceed thrust, and proton fusion loses a billion times more energy than it generates.

Mitophagy—your cells' cleanup mechanism for damaged mitochondria—holds the key to preventing Parkinson's, Alzheimer's, heart disease, and diabetes. Scientists have discovered you can boost this process through exercise, fasting, and specific compounds like spermidine and urolithin A.

Shifting baseline syndrome explains why each generation accepts environmental degradation as normal—what grandparents mourned, you take for granted. From Atlantic cod populations that crashed by 95% to Arctic ice shrinking by half since 1979, humans normalize loss because we anchor expectations to our childhood experiences. This amnesia weakens conservation policy and sets inadequate recovery targets. But tools exist to reset baselines: historical data, long-term monitoring, indigenous knowle...

Social media has created an 'authenticity paradox' where 5.07 billion users perform carefully curated spontaneity. Algorithms reward strategic vulnerability while psychological pressure to appear authentic causes creator burnout and mental health impacts across all users.

Scientists have decoded how geckos defy gravity using billions of nanoscale hairs that harness van der Waals forces—the same weak molecular attraction that now powers climbing robots on the ISS, medical adhesives for premature infants, and ice-gripping shoe soles. Twenty-five years after proving the mechanism, gecko-inspired technologies are quietly revolutionizing industries from space exploration to cancer therapy, though challenges in durability and scalability remain. The gecko's hierarch...

Cities worldwide are transforming governance through digital platforms, from Seoul's participatory budgeting to Barcelona's open-source legislation tools. While these innovations boost transparency and engagement, they also create new challenges around digital divides, misinformation, and privacy.

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.