Epigenetic Clocks Predict Disease 30 Years Early

TL;DR: Genetically engineered pig organs are now functioning in human patients for months after decades of failures, thanks to CRISPR technology that eliminates immune rejection triggers. FDA-approved trials signal the technology could solve organ shortages by 2030, though ethical, economic, and access challenges remain.
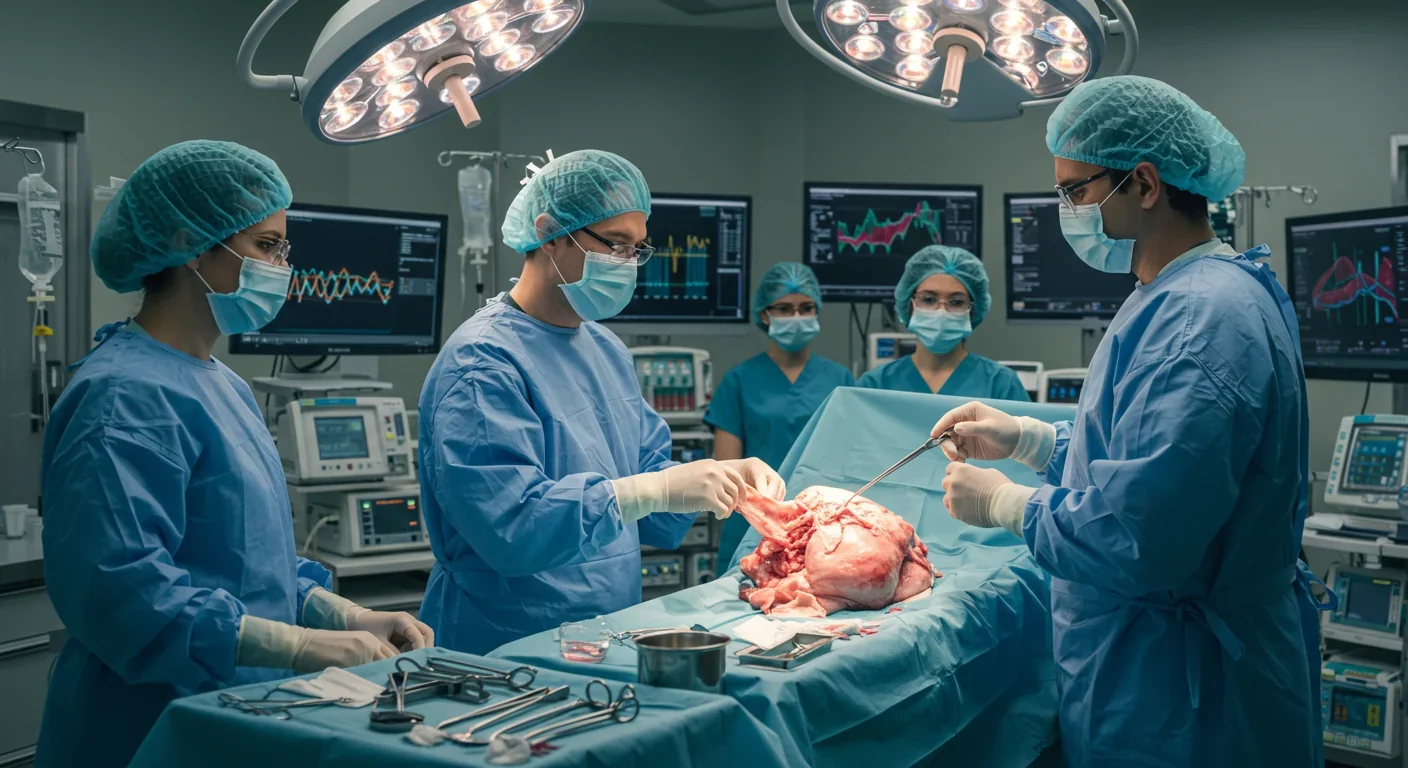
By 2030, patients dying on transplant waiting lists could become a relic of medical history. That's not hyperbole—it's the trajectory we're on right now. After decades of failed experiments and ethical debates, genetically engineered pig organs are functioning inside human bodies, not just for days or weeks, but for months. The FDA has approved clinical trials. Patients are going home. And the implications stretch far beyond medicine, into questions about what it means to be human, who gets access to life-saving technology, and how we'll rewrite the social contract around healthcare in the coming decades.
In September 2025, Tim Andrews became the first person to survive more than 222 days with a genetically modified pig kidney functioning in his body. A second patient, Bill Stewart, reached 82 days. Both were discharged shortly after surgery and are living relatively normal lives. These aren't experimental procedures performed on the terminally ill as a last resort anymore—they're FDA-approved clinical trials designed to gather data on safety and efficacy across multiple transplant centers.
The numbers tell the story. More than 800,000 Americans suffer from end-stage kidney disease. In 2024, only about 28,000 human kidneys were transplanted. That gap isn't closing through traditional donation—it's widening. Every day, 17 people die waiting for an organ that never arrives. Xenotransplantation doesn't just offer hope; it offers scalability. Pigs reach maturity quickly, their organs are similar in size to ours, and they can be bred in controlled environments.
What makes this possible isn't willpower or wishful thinking—it's CRISPR-Cas9 gene editing, the technology that won its inventors a Nobel Prize. Scientists can now make precise changes to pig DNA, removing the biological incompatibilities that caused every previous attempt to fail spectacularly.
For most of medical history, putting a pig organ into a human body was a guaranteed death sentence. The immune system would attack within minutes, triggering what's called hyperacute rejection—a violent response to foreign proteins on the surface of pig cells. Patients would die on the operating table.
The solution required rewriting pig genetics from the ground up. EGEN-2784, the lead candidate from eGenesis, carries three classes of genetic modifications. First, scientists knocked out three genes that produce glycan antigens—the molecular flags that trigger immediate immune attack. Specifically, they eliminated the α-Gal epitope by inactivating the GGTA1 gene, along with CMAH and B4GALNT2, which produce other xenoantigens.
Second, they inserted seven human genes into the pig genome. These aren't random additions—they're carefully selected proteins that regulate immune response, reduce inflammation, improve blood clotting compatibility, and control complement activation. It's like giving the pig organ a diplomatic passport so the human immune system recognizes it as "self" rather than invader.
Third, and perhaps most impressively, they inactivated porcine endogenous retroviruses—ancient viral sequences embedded in pig DNA that could, theoretically, infect human cells. Using multiplex CRISPR editing, scientists disrupted dozens of PERV sequences simultaneously, something impossible with older genetic engineering methods.
The result is an organ that looks like a pig kidney but functions in a human body without immediate rejection. It's a feat of precision engineering that would have been science fiction a decade ago.
The first pig-to-human heart transplants happened in 2022 and 2023 at the University of Maryland Medical Center, using organs with ten gene edits. The patients didn't survive long-term, but the organs functioned for weeks—proof of concept that the technology wasn't fundamentally flawed.
Kidney transplants have been more successful. Massachusetts General Hospital performed a landmark procedure in 2024, and NYU Langone followed with another in November of that year. The FDA's approval of eGenesis's Phase I/II/III trial in 2025 marked a shift from one-off compassionate use cases to systematic clinical evaluation. The trial will enroll 33 patients across multiple centers, with Tim Andrews and Bill Stewart already past the initial 12-week sentinel period without major complications.
Even more experimental work is happening with other organs. In August 2025, a genetically engineered pig lung with six gene edits provided ventilatory support for nine days without hyperacute rejection, though antibody-mediated injury appeared after day three. Corneas, livers, and even pancreatic islet cells are being tested in various stages of development.
The clinical picture isn't perfect. Patients still require immunosuppression drugs, just like with human organ transplants. There are unknowns about long-term function—will these organs last five years? Ten? Twenty? And while the initial results are promising, we're still in early innings. But the trajectory is clear: this technology works, and it's improving rapidly.

We've been here before, in a way. When the first human heart transplant was performed in 1967, it was considered radical, even sacrilegious. The patient lived 18 days. Critics called it a publicity stunt. Now, heart transplants are routine, and patients live for decades.
Xenotransplantation follows a similar arc, but with a twist—we're not just transplanting organs, we're engineering them. That puts this breakthrough in the lineage of GMO crops, synthetic insulin, and monoclonal antibodies: technologies where humans directly edit living systems to serve our needs.
The history of xenotransplantation is littered with failures. Early 20th-century attempts using primate organs failed catastrophically. In 1984, Baby Fae received a baboon heart and lived 21 days before dying of rejection. For decades, the field was stuck—every attempt ended the same way, with the immune system destroying the foreign organ.
What changed wasn't just technology, but our understanding of immunology at the molecular level. We didn't just need better surgical techniques; we needed to rewrite the biological code. CRISPR gave us that power. It's the difference between trying to translate a book by guessing and having a dictionary that lets you change any word with precision.
The lesson from history is that breakthroughs in medicine often seem impossible until they're inevitable. Antibiotics, vaccines, in vitro fertilization—all were controversial, all faced ethical objections, and all became standard care. Xenotransplantation is following the same path.
Creating a transplantable pig organ isn't like breeding dogs for specific traits. It requires industrial-scale genetic engineering, specialized animal husbandry, and rigorous biosecurity protocols.
Companies like eGenesis and Revivicor maintain herds of genetically modified pigs in controlled facilities. These aren't farm animals—they're living biomedical products, raised in pathogen-free environments to prevent any risk of disease transmission. Each pig is essentially a clone, genetically identical to ensure consistency in organ quality.
The genetic modifications are made at the embryonic stage. Scientists use CRISPR to edit pig embryos, implant them in surrogate mothers, and raise the offspring under strict conditions. It takes months to produce a single transplantable organ, and the process requires significant investment in infrastructure and expertise.
But here's what makes it scalable: unlike human organ donation, which depends on tragedy and altruism, pig organ production can be planned, optimized, and expanded. If demand increases, you breed more pigs. If a new genetic modification improves outcomes, you update the breeding stock. It's manufacturing applied to biology, and that predictability changes everything.
The organs aren't perfect replicas of human tissue—they're hybrid constructs, part pig and part human at the genetic level. That raises philosophical questions (is it still a pig organ if it carries human genes?) and practical ones (how do we regulate something that's neither fully animal nor fully human?).
If xenotransplantation becomes routine, it doesn't just save lives—it disrupts healthcare economics, ethics, and social structures around organ allocation.
Right now, organ transplantation is governed by scarcity. Who gets on the waiting list, who moves up, who gets priority—these are life-and-death decisions shaped by age, health status, and sometimes luck. If we suddenly have abundant organs, those systems collapse. Do we still prioritize the sickest patients, or do we shift to maximizing total life-years saved? Do we charge for pig organs like we charge for other medical devices, or treat them as public goods?
Access will be uneven at first. Clinical trials happen in major medical centers, often in wealthy countries. Even when approved, xenotransplants will likely be expensive initially, raising the specter of a two-tier system where the rich get pig organs and the poor wait for human donors.
Ethical debates center on animal welfare, religious objections, and cultural acceptance. Some Muslim and Jewish communities have raised concerns about using pigs, though theological interpretations vary when life-saving treatment is at stake. Animal rights advocates question whether it's ethical to breed pigs specifically for organ harvest, even if they're treated humanely.
There's also the question of informed consent. Patients receiving pig organs are pioneers, and while they're desperate, they're also taking unknown risks. What if a dormant pig virus activates years later? What if there are immunological complications we haven't predicted? The first generation of recipients are, in a real sense, test subjects.
But the alternative—letting people die on waiting lists—has its own moral weight. If the technology works, withholding it becomes harder to justify. We'll have to navigate these tensions in real time, making policy as the science evolves.

Xenotransplantation isn't just an American or European story. South Korea is investing heavily in the technology, viewing it as both a healthcare solution and an export opportunity. China has conducted its own preclinical trials and is developing domestic capabilities. Even countries with lower healthcare budgets see the potential—if pig organs become affordable, they could leapfrog traditional transplant infrastructure entirely.
International cooperation will be essential. Regulatory standards need to be harmonized so that organs produced in one country can be used in another. Biosecurity protocols must be rigorous to prevent cross-species disease transmission. And ethical guidelines should reflect diverse cultural values, not just Western bioethical frameworks.
There's also competition. Companies like eGenesis, Revivicor, and others are racing to commercialize the technology, filing patents and forming partnerships with hospitals. Whoever dominates the market will shape how xenotransplantation develops globally—whether it's affordable and accessible, or expensive and exclusive.
If xenotransplantation succeeds, the ripple effects will extend far beyond transplant wards. Medical education will need to incorporate xenobiology. Regulatory agencies will need new frameworks for approving hybrid biological products. Insurance companies will have to decide how to price and cover these procedures.
For individuals, it means rethinking what we mean by "natural" and "human." If you have a pig kidney, are you still fully human? Most people would say yes, but the question isn't trivial—it touches on identity, bodily autonomy, and the boundaries of medical intervention.
There are also skills to develop. Surgeons need training in xenotransplant procedures. Immunologists need to understand the unique rejection profiles of cross-species organs. Ethicists, policymakers, and patient advocates need to stay ahead of the technology, anticipating problems before they become crises.
We're entering an era where biology is engineered, not just studied. Xenotransplantation is one piece of that puzzle, alongside synthetic biology, gene therapy, and AI-designed drugs. Together, they represent a shift from reactive medicine (treating disease after it occurs) to proactive bioengineering (designing solutions at the molecular level).
The challenge isn't just making the technology work—it's making it work for everyone.
The next five years will be critical. If current trials succeed, we'll see FDA approval for commercial xenotransplants by 2028 or 2029. That approval will trigger a cascade: insurance coverage, hospital adoption, international regulatory alignment, and public normalization.
If trials falter—if long-term complications emerge, if rejection rates remain too high, if a biosecurity incident occurs—the field could face another winter, like it did after the failures of the 1980s and 1990s.
But the momentum feels different this time. The science is more mature, the clinical results are more promising, and the need is more urgent. Transplant waiting lists aren't shrinking, and human organ donation rates have plateaued. Xenotransplantation isn't just a nice-to-have—it's becoming a necessity.
For patients like Tim Andrews and Bill Stewart, the future is already here. They're living proof that the boundary between human and animal, between natural and engineered, is more permeable than we thought. And that's both thrilling and unsettling.
What we do with this technology in the next decade will reveal a lot about who we are as a species—whether we can distribute medical miracles equitably, whether we can balance innovation with ethics, and whether we can adapt to a world where biology itself is just another form of technology.
The organ shortage isn't solved yet. But for the first time, we can see the path to solving it. And that path runs through a pig farm, a CRISPR lab, and an operating room where the impossible is becoming routine.

Recent breakthroughs in fusion technology—including 351,000-gauss magnetic fields, AI-driven plasma diagnostics, and net energy gain at the National Ignition Facility—are transforming fusion propulsion from science fiction to engineering frontier. Scientists now have a realistic pathway to accelerate spacecraft to 10% of light speed, enabling a 43-year journey to Alpha Centauri. While challenges remain in miniaturization, neutron management, and sustained operation, the physics barriers have ...

Epigenetic clocks measure DNA methylation patterns to calculate biological age, which predicts disease risk up to 30 years before symptoms appear. Landmark studies show that accelerated epigenetic aging forecasts cardiovascular disease, diabetes, and neurodegeneration with remarkable accuracy. Lifestyle interventions—Mediterranean diet, structured exercise, quality sleep, stress management—can measurably reverse biological aging, reducing epigenetic age by 1-2 years within months. Commercial ...

Data centers consumed 415 terawatt-hours of electricity in 2024 and will nearly double that by 2030, driven by AI's insatiable energy appetite. Despite tech giants' renewable pledges, actual emissions are up to 662% higher than reported due to accounting loopholes. A digital pollution tax—similar to Europe's carbon border tariff—could finally force the industry to invest in efficiency technologies like liquid cooling, waste heat recovery, and time-matched renewable power, transforming volunta...

Humans are hardwired to see invisible agents—gods, ghosts, conspiracies—thanks to the Hyperactive Agency Detection Device (HADD), an evolutionary survival mechanism that favored false alarms over fatal misses. This cognitive bias, rooted in brain regions like the temporoparietal junction and medial prefrontal cortex, generates religious beliefs, animistic worldviews, and conspiracy theories across all cultures. Understanding HADD doesn't eliminate belief, but it helps us recognize when our pa...

The bombardier beetle has perfected a chemical defense system that human engineers are still trying to replicate: a two-chamber micro-combustion engine that mixes hydroquinone and hydrogen peroxide to create explosive 100°C sprays at up to 500 pulses per second, aimed with 270-degree precision. This tiny insect's biochemical marvel is inspiring revolutionary technologies in aerospace propulsion, pharmaceutical delivery, and fire suppression. By 2030, beetle-inspired systems could position sat...

The U.S. faces a catastrophic care worker shortage driven by poverty-level wages, overwhelming burnout, and systemic undervaluation. With 99% of nursing homes hiring and 9.7 million openings projected by 2034, the crisis threatens patient safety, family stability, and economic productivity. Evidence-based solutions—wage reforms, streamlined training, technology integration, and policy enforcement—exist and work, but require sustained political will and cultural recognition that caregiving is ...

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.