How Compassion Meditation Rewires Your Brain & Cuts Inflammation

TL;DR: Naked mole rats survive 18 minutes without oxygen by switching to fructose metabolism, a plant-like pathway that could revolutionize treatment for strokes, cardiac arrest, and organ preservation in humans.
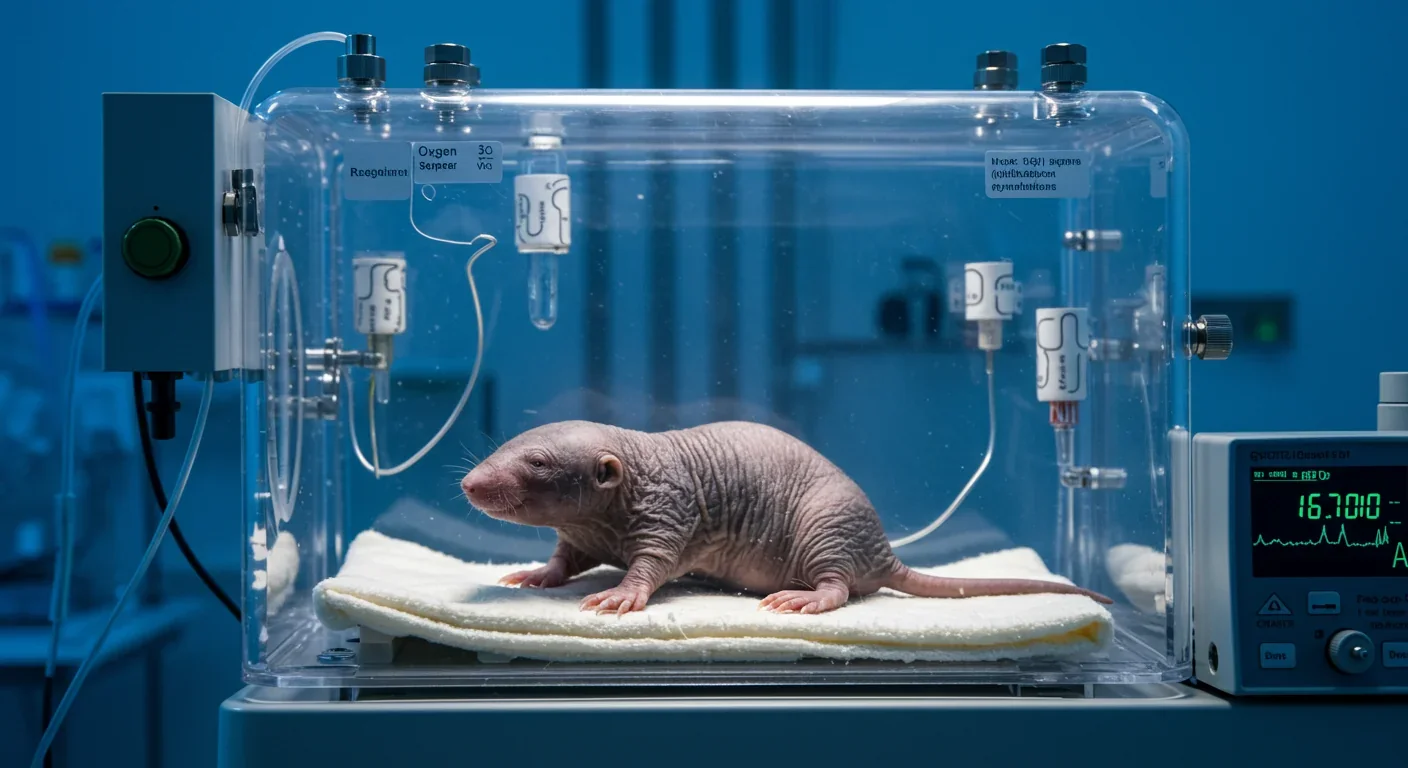
Imagine your brain cells continuing to function normally even when deprived of oxygen for 18 minutes straight. For humans, that scenario means certain death. Brain cells start dying within three to five minutes without oxygen. But for the naked mole rat, a peculiar rodent that lives in underground colonies beneath East Africa, 18 minutes without oxygen is just another Tuesday.
This isn't science fiction. It's evolutionary engineering at its finest, and researchers believe these bizarre creatures might hold the key to preventing brain damage from strokes, surviving cardiac arrest, and keeping donor organs viable longer during transplantation. The implications stretch far beyond biology labs. We're talking about rewriting the rules of emergency medicine.
Naked mole rats are, to put it mildly, weird. They're nearly hairless, almost blind, and live in underground burrow systems that can house up to 300 individuals. Their colonies operate like insect hives, complete with a single breeding queen and workers who never reproduce. They're also essentially immune to cancer, resistant to pain, and can live over 30 years—ten times longer than similarly sized rodents.
But their most astonishing trait is their tolerance for oxygen deprivation. In laboratory tests, these animals survived 18 minutes in zero-oxygen conditions without apparent harm. They also thrive in environments with CO2 concentrations that would kill most mammals, tolerating atmospheres with 80% carbon dioxide and just 20% oxygen.
Their underground lifestyle explains this adaptation. Sealed burrows with hundreds of animals produce dangerously high CO2 levels and depleted oxygen. Evolution didn't give naked mole rats better ventilation systems. Instead, it fundamentally rewired their metabolism.
The breakthrough came in 2017, when researchers at the University of Illinois at Chicago discovered something unprecedented: naked mole rats can switch their metabolism to run on fructose instead of glucose when oxygen runs out. Think of it as installing an emergency backup generator that runs on entirely different fuel.
Here's why that matters. Every mammal's brain depends on glucose for energy, and glucose metabolism requires oxygen. Cut off the oxygen supply, and brain cells quickly exhaust their energy reserves and die. It's a fundamental biological constraint that evolution seemed unable to overcome—until naked mole rats came along.
When oxygen levels plummet, these animals activate fructose-driven glycolysis, a metabolic pathway normally seen only in plants and yeast. Fructose can be broken down without oxygen to produce energy, though not as efficiently as the standard glucose pathway. The naked mole rat's cells pump fructose into the brain using GLUT-5 transporters, molecular pumps that in all other mammals exist only in intestinal cells.
This metabolic shift comes with a price. The animals enter a state of suspended animation, slowing their pulse and breathing dramatically while reducing movement to near zero. They're essentially putting themselves into a reversible coma to ride out the oxygen shortage.
But there's a catch with fructose metabolism: it produces lactic acid, which causes acidosis—a dangerous buildup of acid in body tissues. In humans and other mammals, acidosis itself inhibits glycolysis, creating a deadly feedback loop. Naked mole rats, however, have evolved resistance to acidosis, allowing them to tolerate the lactic acid buildup that would kill other animals.
The fructose trick is just one piece of the puzzle. These animals have also optimized their oxygen delivery systems. Their hemoglobin, the protein that carries oxygen in blood, has evolved to have exceptionally high affinity for oxygen. This means their blood can extract and hold onto oxygen molecules even when ambient oxygen levels are extremely low.
Their baseline metabolic rate is about 70% that of a mouse, meaning they simply need less oxygen to function in the first place. It's like running an energy-efficient appliance versus a power-hungry one—when the electricity gets scarce, the efficient model keeps working longer.
Recent research has uncovered even more protective mechanisms. A 2024 study published in Nature Communications revealed that naked mole rat heart cells have unique gene expression patterns that control energy generation from sugars. During simulated heart attacks in laboratory conditions, their hearts maintained enhanced energy reserves even when blood flow was cut off and then restored.
The same study found that these cardiac adaptations result in negligible tissue damage during hypoxic events that would cause significant injury in human hearts. While humans face serious risk of permanent heart damage from even brief oxygen deprivation during cardiac arrest, naked mole rat hearts essentially shrug off the insult.

For decades, scientists assumed that hypoxia-tolerant animals protected their brains through a mechanism called "channel arrest"—essentially shutting down the cellular channels that allow calcium ions to flood into oxygen-starved neurons. Calcium overload is one of the main ways that brain cells die during strokes and cardiac arrest.
But a 2025 study turned that assumption upside down. Researchers measuring calcium movements in naked mole rat brain slices found that channel arrest doesn't happen at all in these animals. During hypoxia, their neurons continued allowing normal calcium flux, yet didn't suffer damage.
So how do they avoid calcium-induced cell death? The research points to enhanced mitochondrial buffering—the cellular power plants in naked mole rat neurons appear to have superior capacity to absorb and sequester excess calcium, preventing it from triggering the death cascade that would normally follow.
This finding matters because it reveals that evolution has found multiple solutions to the hypoxia tolerance problem. Channel arrest isn't the only path forward, which expands the potential strategies researchers might pursue when developing therapies for human oxygen-deprivation injuries.
Other protective factors have emerged from recent studies. MicroRNA profiling revealed that 18 specific microRNAs change their expression in naked mole rat brains during hypoxia. These small RNA molecules regulate gene expression and may help coordinate the cellular response to oxygen deprivation.
Naked mole rats aren't the only animals that laugh at oxygen deprivation. Turtles can survive months without oxygen while hibernating in frozen ponds. Wood frogs freeze solid during winter, their hearts stopping completely, then thaw out in spring and hop away. Certain fish can enter cryptobiosis—a state of suspended animation where metabolic activity essentially stops—and revive when conditions improve.
Each species has evolved its own toolkit for handling extreme oxygen stress. Turtles release massive amounts of calcium from their shells to buffer the lactic acid produced during anaerobic metabolism. Some fish produce specialized proteins that protect cellular structures during prolonged oxygen deprivation. Comparing these different strategies gives researchers a broader palette of potential approaches to protecting human tissues.
What makes naked mole rats particularly valuable for medical research is that they're mammals. Their basic physiology is much closer to ours than that of turtles or fish, making it more likely that insights from naked mole rat biology can translate to human therapies. They've essentially shown that mammalian brains can be protected from oxygen deprivation—evolution just hasn't bothered to install those protections in humans because we haven't needed them.
Every year, hundreds of thousands of people suffer strokes, which occur when blood flow to part of the brain is interrupted. The standard treatment window is extremely narrow—doctors have just a few hours to restore blood flow before brain damage becomes permanent. If researchers could develop drugs that trigger fructose metabolism or enhance mitochondrial calcium buffering in human neurons, that treatment window might expand dramatically.
Cardiac arrest presents a similar challenge. When the heart stops, brain cells begin dying within minutes. Survivors often face permanent cognitive impairment because of this oxygen deprivation. Understanding how naked mole rat hearts maintain energy reserves during blood flow interruption could lead to better preservation techniques during cardiac surgery or improved resuscitation protocols.
Organ transplantation is another frontier. Donor organs deteriorate rapidly once removed from the body, limiting how long they can be preserved and how far they can be transported. Current preservation methods keep organs viable for just hours, which restricts the donor pool and forces difficult logistical compromises. If scientists could apply naked mole rat metabolic strategies to preserve human organs, transplant medicine would be revolutionized.
Dr. Dunja Aksentijevic, who led the cardiac metabolism research, noted that unlike humans who are prone to heart injury from oxygen deprivation, naked mole rat hearts have adapted to completely evade such damage. The challenge now is identifying which specific molecular mechanisms are responsible and determining whether they can be pharmacologically activated in humans.
Some researchers are exploring whether the GLUT-5 transporter could be temporarily activated in human brain cells during medical emergencies. Others are investigating the signaling pathways that trigger the naked mole rat's suspended animation state, wondering if inducing a controlled, reversible metabolic slowdown might protect trauma patients during transport or surgery.

This isn't a single lab pursuing a quirky interest in weird rodents. Research teams across multiple continents are racing to decode naked mole rat biology. The University of Illinois at Chicago, Queen Mary University of London, and institutions across Europe and Asia are collaborating to map out the complete picture of how these animals achieve their remarkable resilience.
The work requires multiple disciplines. Geneticists sequence and compare genomes to identify the specific mutations that enable hypoxia tolerance. Physiologists measure metabolic parameters in real time during oxygen deprivation. Neurologists study calcium dynamics in living brain tissue. Cardiac researchers analyze gene expression patterns in heart cells. Each piece of the puzzle contributes to the larger understanding.
International scientific conferences now regularly feature sessions on comparative hypoxia tolerance, where researchers share findings about naked mole rats, turtles, diving mammals, and other oxygen-deprived champions. The goal isn't just to understand these animals for their own sake—it's to create a comprehensive catalog of biological strategies that evolution has invented to solve the oxygen deprivation problem.
This global cooperation accelerates progress. When one team discovers a new mechanism, others can immediately begin testing whether similar pathways exist in other species or exploring how to activate them pharmacologically. The open exchange of data and techniques has turned what might have been decades of isolated efforts into a coordinated scientific campaign.
Several research groups have moved from basic discovery toward potential applications. While no naked mole rat-inspired drugs are in clinical trials yet, preclinical research is advancing on multiple fronts.
One approach involves developing small molecule drugs that could temporarily activate fructose metabolism in human cells during emergencies. Another focuses on compounds that might enhance mitochondrial calcium buffering capacity. A third investigates whether the microRNAs that change during naked mole rat hypoxia could be delivered therapeutically to protect human brain tissue.
The pharmaceutical development process is notoriously slow and expensive, especially for treatments targeting acute conditions like stroke and cardiac arrest. Clinical trials must demonstrate both safety and efficacy, often requiring years and hundreds of millions of dollars. But the potential payoff is enormous—even modest improvements in outcomes for stroke and cardiac arrest would save millions of lives globally.
Beyond pharmaceuticals, some researchers are exploring whether the insights could improve medical devices. Could a bypass machine be redesigned to provide fructose to the brain during cardiac surgery? Might organ preservation solutions be reformulated to mimic the metabolic conditions in naked mole rat tissues?
Translating these findings to human medicine faces significant challenges. The naked mole rat's adaptations evolved over millions of years and involve complex interactions between multiple genes and cellular systems. You can't just flip a single switch and make human cells hypoxia-tolerant.
There's also the issue of unintended consequences. The metabolic pathways that protect naked mole rats during oxygen deprivation might have side effects if activated in humans. Fructose metabolism produces less energy than glucose metabolism—fine for an animal entering suspended animation, but potentially problematic for maintaining normal human brain function.
Safety concerns loom large. Any drug that fundamentally alters cellular metabolism or calcium handling needs extensive testing to ensure it doesn't cause more problems than it solves. The regulatory bar for emergency medical interventions is appropriately high, given that patients would be receiving these treatments during life-threatening crises.
Yet the research community remains optimistic. As one investigator noted, the naked mole rat has simply rearranged basic building blocks of metabolism to achieve super-tolerance to low oxygen conditions. If evolution can do it through random mutations and natural selection, perhaps targeted drug development can achieve similar results through rational design.
The implications extend beyond emergency medicine. If researchers crack the code on preventing hypoxic brain injury, it could change how we approach aging, since oxygen deprivation plays a role in various neurodegenerative diseases. It might enable new surgical techniques that require temporarily stopping blood flow to the brain. It could allow humans to function better at extreme altitudes or in other low-oxygen environments.
Think about the skills this research is already creating. A new generation of scientists is learning to work at the intersection of comparative physiology, molecular biology, and translational medicine. They're developing expertise in metabolic engineering, drug design, and cross-species genetic analysis. These capabilities will prove valuable far beyond the specific challenge of hypoxia tolerance.
For medical students and researchers, this field offers a masterclass in how evolution can inspire innovation. Nature has already conducted billions of experiments through millions of years. The results are walking, swimming, and burrowing all around us. Learning to read that biological archive and translate it into human applications may be one of the most powerful tools in 21st-century medicine.
The next decade will likely bring more surprises from naked mole rat research. We're still in the early stages of understanding these animals. Each study that answers one question raises three new ones. What other protective mechanisms remain undiscovered? How do all these systems coordinate during oxygen deprivation? Can the same mechanisms that prevent acute hypoxic injury also address chronic conditions?
Funding agencies worldwide are taking notice. Government research organizations and private foundations are directing resources toward comparative hypoxia research. The potential applications in stroke, cardiac arrest, trauma medicine, and organ preservation justify significant investment.
Within the next few years, expect to see the first clinical trials testing whether naked mole rat-inspired interventions can protect human tissues. These initial studies will likely be small, carefully controlled, and focused on safety. But they'll represent a crucial milestone—the moment when insights from peculiar underground rodents move from laboratory curiosities to potential lifesaving therapies.
The broader lesson is about maintaining scientific curiosity. Naked mole rats were dismissed for decades as ugly, weird rodents with no practical importance. Now they're at the frontier of medical innovation. This pattern repeats throughout scientific history—seemingly esoteric studies of strange organisms often yield the most transformative discoveries.
Naked mole rats are teaching us that biological constraints we thought were absolute are actually negotiable. Mammals can survive extended oxygen deprivation. Brains can protect themselves from calcium overload without channel arrest. Hearts can maintain energy reserves even when blood flow stops. These aren't magic tricks—they're sophisticated biological engineering solutions that evolution has already field-tested.
The question isn't whether naked mole rat research will impact human medicine. It already has, by expanding our understanding of what's physiologically possible. The question is how soon those insights translate into actual therapies that paramedics can administer, that emergency room doctors can prescribe, that surgeons can rely on.
We're witnessing the early chapters of what could be a revolution in how we handle medical emergencies involving oxygen deprivation. The naked mole rat has shown us the destination. Now it's up to researchers to map the route and build the vehicles that will get us there.
For anyone whose life might one day be saved by an extra 15 minutes of protection against oxygen deprivation—which, given how common strokes and cardiac arrests are, could be almost anyone—these weird little rodents deserve our attention and gratitude. They're not just surviving in their underground burrows. They're quietly rewriting the future of medicine.

Europa and Enceladus host ice volcanoes that erupt water and organic molecules from subsurface oceans into space, creating natural sampling opportunities for detecting extraterrestrial life. NASA missions are revolutionizing our search.

Loving-kindness meditation produces measurable brain changes in as little as eight weeks—increasing gray matter in empathy regions while reducing amygdala volume—and simultaneously lowers inflammatory markers like IL-6 and CRP by boosting vagal tone, making compassion practice a scientifically validated intervention with both neurological and immune system benefits.

Scientists propose deploying massive underwater curtains to block warm ocean currents from melting vulnerable glaciers. While the technology could theoretically slow ice sheet collapse, it faces enormous engineering challenges, ecological risks, and costs up to $500 billion—raising questions about whether it represents genuine climate action or a dangerous distraction from emissions reduction.

Religious communities worldwide engage in painful rituals because suffering functions as an impossible-to-fake signal of commitment. These costly displays filter out free-riders, create physiological synchrony between participants, and forge intense social bonds through shared sacrifice.

Scientists have discovered that plants generate electrical signals remarkably similar to nerve impulses, using calcium-based action potentials to coordinate growth, defend against threats, and communicate across their entire bodies without any nervous system—a finding that's revolutionizing agriculture and our understanding of plant intelligence.

Social media algorithms aren't just showing you content—they're reshaping your beliefs. New research reveals how recommendation systems push users toward extremism through engagement-driven feedback loops, exploiting psychological biases while gradually radicalizing worldviews.

Zero-knowledge proofs let you prove facts about yourself without revealing the underlying data, transforming digital privacy across identity verification, authentication, blockchain, healthcare, voting, and finance through cryptographic protocols that separate verification from disclosure.