Underground Air Storage: Renewable Energy's Hidden Battery

TL;DR: Over 12.8 million farming families now grow biofortified crops—staple foods bred or engineered to contain higher levels of essential vitamins and minerals. These crops are proving cost-effective at fighting hidden hunger, with evidence showing $17 in benefits for every dollar invested and measurable health improvements in vulnerable populations across Africa, Asia, and Latin America.

In 2024, over 12.8 million farming families across 30 countries are growing something revolutionary. Not a new gadget or app that promises to change the world. Something simpler, older, and far more powerful: food that fights malnutrition from the inside out.
These families are cultivating biofortified crops—staple foods like rice, beans, and sweet potatoes that have been bred or engineered to pack significantly higher concentrations of essential vitamins and minerals. It's agriculture meeting nutrition science, and the results are quietly transforming public health in ways that supplements and fortified processed foods never could.
The numbers tell a stark story. More than 2 billion people worldwide suffer from "hidden hunger"—they eat enough calories to survive but lack the micronutrients their bodies need to truly thrive. Iron deficiency alone affects women and children across Africa, Asia, and Latin America, causing anemia, cognitive impairment, and reduced work capacity. Vitamin A deficiency blinds hundreds of thousands of children each year. Zinc deficiency stunts growth and weakens immune systems.
Traditional solutions—vitamin pills, food fortification at processing plants, supplementation campaigns—help, but they're expensive, require infrastructure, and often don't reach the rural poor who need them most. Biofortification offers a different path: boost the nutrients in the crops people already grow and eat every day. The food becomes the delivery system.
Biofortification works through three main approaches, each with distinct advantages and controversies.
Conventional breeding takes advantage of natural genetic variation. Plant breeders identify varieties with higher micronutrient content, cross them, and select offspring that combine high nutrition with good yield, taste, and disease resistance. It's the same technique humans have used for millennia, just turbocharged with modern genomics. This method faces fewer regulatory hurdles and enjoys broader public acceptance, but it's limited to traits already present within a species.
Genetic modification goes further, inserting genes from other organisms to create nutrient profiles impossible through breeding alone. The most famous example is Golden Rice, which contains genes from a soil bacterium and maize that enable the rice grain to produce beta-carotene, a precursor to vitamin A. Standard white rice has essentially zero vitamin A content. Golden Rice glows with it.
Biofortification embeds nutrition into the crop itself, making it accessible to subsistence farmers without requiring industrial infrastructure or supply chains.
Agronomic biofortification takes a third route: applying micronutrient-rich fertilizers (especially zinc, selenium, or iodine) to crops during growth so they absorb and accumulate higher levels in edible parts. It's faster and cheaper than breeding but requires farmers to purchase and apply these fertilizers season after season.
Each technique has been deployed successfully across different crops and contexts. Iron beans in Rwanda. Vitamin A-enriched orange sweet potatoes in Mozambique and Uganda. High-zinc wheat in India and Pakistan. Pearl millet with enhanced iron and zinc across West Africa. Orange maize released in 11 countries and under trial in 23 more.

Skeptics might wonder whether breeding higher nutrient levels into crops translates to better health outcomes in real people. The evidence from randomized controlled trials and population studies is increasingly convincing.
In Mozambique, children who ate beta-carotene-rich orange sweet potatoes showed a 24 percent reduction in vitamin A deficiency compared to those eating conventional white varieties. Two separate trials in India found that children consuming iron- and zinc-biofortified pearl millet improved their iron status and hemoglobin concentrations, especially among those who were deficient at baseline.
Iron beans developed by HarvestPlus and partners contain up to 90 percent more iron than standard varieties—jumping from about 5 mg per 100 grams to nearly 9.5 mg. When eaten regularly, they provide up to 80 percent of daily iron needs for women and children. Studies show they don't just boost iron status; they also improve memory, attention, and physical work capacity in young women. These aren't abstract lab results. They're measurable improvements in people's lives.
"For every USD invested in biofortification, they estimate as much as 17 USD of benefits gained."
— Copenhagen Consensus Center
The Copenhagen Consensus Center, which ranks global development interventions by cost-effectiveness, estimates that for every dollar invested in biofortification, as much as $17 in benefits are gained—measured in reduced healthcare costs, improved productivity, and lives saved. The cost per disability-adjusted life year (DALY) saved ranges from $15 to $20, far below the World Bank's threshold of $270 for highly cost-effective interventions.
Technology means nothing if it sits on a shelf. Biofortification only works if farmers choose to plant these varieties and families choose to eat them.
The track record so far is encouraging. Between 2004 and 2021, the number of smallholder farming families growing biofortified crops exploded from near zero to 12.8 million. In Rwanda, just four years after iron beans were introduced, they accounted for nearly 12 percent of national bean production. By the end of the HarvestPlus project there, 15 percent of the entire Rwandan population was consuming them regularly.
Why? Because agronomic performance matters as much as nutrition. Iron bean varieties aren't just higher in micronutrients; they're also high-yielding, disease-resistant, and have taste and cooking qualities farmers and consumers prefer. If a biofortified variety underperforms its conventional counterpart in the field or the kitchen, it won't spread no matter how nutritious it is.
Consumer acceptance studies show that when people understand the health benefits, they're willing to pay more for biofortified foods. In randomized trials in Uganda and Mozambique, adoption rates for vitamin A orange sweet potatoes exceeded 60 percent, with sustained consumption over time.

Still, cultural preferences matter. In parts of Africa, white maize is preferred for human consumption while yellow maize is associated with animal feed or food aid—a perception that initially hindered acceptance of orange, vitamin A-enriched maize. Color matters. Trials found that rural populations will eat biofortified varieties even if they differ in appearance, but education about nutritional benefits is essential. Taste tests, cooking demonstrations, and peer influence all play roles in shifting norms.
No discussion of biofortification's future can ignore Golden Rice, arguably the world's most famous—and most controversial—biofortified crop.
Developed in the late 1990s, Golden Rice was engineered to produce beta-carotene in the rice grain, addressing vitamin A deficiency that blinds and kills hundreds of thousands of children annually in Asia, where rice is the staple. The humanitarian intention was clear. The technology worked. And then it spent more than two decades tangled in regulatory, political, and activist opposition.
Golden Rice finally received approval for commercial cultivation in the Philippines in 2021—more than 20 years after its development. That delay represents untold preventable suffering. Critics raised concerns about GMO safety, corporate control of seeds, and whether biofortification would distract from broader food system reforms. Supporters argued that perfect shouldn't be the enemy of good, and that children were going blind while activists debated.
Golden Rice spent over 20 years in regulatory review while hundreds of thousands of children suffered preventable blindness from vitamin A deficiency.
The Golden Rice saga highlights a painful tension: genetically engineered biofortification can achieve nutrient levels impossible through conventional breeding, but it faces regulatory barriers and public skepticism that slow deployment to a crawl. Meanwhile, conventionally bred biofortified crops move faster through the pipeline but can't always reach target nutrient thresholds.
Biofortification exists alongside two other major strategies for addressing micronutrient deficiencies: industrial fortification (adding nutrients to processed foods like flour, salt, or cooking oil) and supplementation (distributing vitamin pills or powders).
Industrial fortification works well in urban areas with centralized food processing and supply chains. It can achieve precise dosing and high nutrient concentrations. Countries like Argentina, Colombia, and the United States have mandatory fortification laws for wheat flour and other staples. Thailand introduced vitamin A-fortified instant noodles in 1994, significantly boosting intake among low-income populations.
But industrial fortification requires infrastructure, regulation, and ongoing costs. It's harder to reach rural populations who grow their own food and rarely buy processed products. There's also a risk of over-fortification if intake isn't monitored.
Supplementation campaigns—distributing high-dose vitamin A capsules twice a year, for instance—can rapidly address acute deficiencies. They've saved countless lives. But they're expensive to maintain, require repeated delivery, and depend on healthcare access. People forget. Supply chains break. Wars and pandemics disrupt distribution.
Biofortification offers something different: a one-time research investment that embeds nutrition into the crop itself. Once farmers adopt biofortified seed, the benefits continue generation after generation with minimal additional cost. It's decentralized, doesn't require infrastructure, and reaches precisely the rural poor who are hardest to reach through other means.
The consensus among nutrition scientists is that all three approaches are complementary, not competitive. Urban populations may benefit most from industrial fortification, while rural subsistence farmers gain most from biofortified crops. Supplementation fills gaps in emergency or high-risk contexts. A mixed strategy tailored to local conditions is most effective.

What enables scientists to pack more nutrients into a grain of rice or a bean?
In conventional breeding, the key is genetic variability. Within any crop species, there's natural variation in micronutrient content. Pearl millet, for example, shows iron levels ranging from 24 to 145 mg per kilogram across different varieties. Breeders screen thousands of lines, identify those with both high nutrients and desirable agronomic traits, and cross them to combine the best of both.
Heritability matters. If a trait like high iron is strongly heritable, selecting for it will reliably produce high-iron offspring. Studies on pearl millet found broad-sense heritability for iron and zinc exceeding 0.67—well above the 0.60 threshold that signals strong selection potential. That means breeders can make rapid progress generation after generation.
There are also fortunate correlations. In many crops, iron and zinc levels are positively correlated (r = 0.58 to 0.81), meaning breeding for one often boosts the other. In iron beans, adding iron also increases zinc content due to co-localization of these minerals in the seed.
"Iron bean consumption also improves women's memory and attention, and ability to perform physical work—essentially skills for productive living."
— HarvestPlus Research
Genetic modification allows even bolder moves. Golden Rice contains genes from Erwinia bacteria and maize that encode enzymes in the beta-carotene biosynthesis pathway. Those enzymes weren't present in rice at all—no amount of traditional breeding could have created them. The result is rice grains that produce beta-carotene at levels sufficient to prevent vitamin A deficiency when eaten regularly.
Agronomic biofortification works because many micronutrients—especially zinc, selenium, and iodine—are readily taken up by plants if present in soil. Applying zinc-enriched fertilizers to wheat or rice at the right growth stages increases grain zinc content significantly. Studies in Ethiopia found that zinc application on wheat could boost grain zinc by 60 percent at costs as low as $2.60 per person per year—a bargain for improved nutrition.
The challenge is ensuring the added nutrients are bioavailable—that human bodies can actually absorb them. Some plant compounds, like phytate, inhibit mineral absorption. Researchers are now breeding low-phytate varieties to improve iron and zinc bioavailability. Others are exploring co-fortification strategies: adding both zinc and selenium, or combining iron with vitamin C, which enhances absorption.
Biofortification doesn't unfold in a vacuum. It bumps up against deeply held beliefs about food, agriculture, nature, and power.
The GMO debate looms largest. While conventionally bred biofortified crops face little opposition, genetically engineered varieties trigger fierce resistance. Critics worry about unknown long-term health effects, environmental risks like gene flow to wild relatives, and the concentration of seed ownership in corporate hands.
Supporters counter that genetic engineering is precise and has an outstanding safety record, with hundreds of studies showing no unique risks compared to conventional breeding. They argue that opposing Golden Rice or other biofortified GMOs on ideological grounds amounts to condemning children to preventable blindness and death.
The intellectual property landscape is complex. Golden Rice was developed by academic researchers and licensed for free to farmers earning less than $10,000 per year. HarvestPlus operates through public-private partnerships, with many biofortified varieties released as open-pollinated or freely available to smallholders. Still, fears persist that biofortification could become a tool for corporate control of agriculture, especially if patents restrict access or lock farmers into proprietary seed systems.

Cultural resistance is real but not insurmountable. People have strong attachments to traditional foods and appearances. In some African communities, white maize is preferred; orange maize seems foreign. In Asia, white rice carries cultural weight; golden-hued rice initially raised suspicion.
Yet field trials consistently show that once people taste biofortified varieties and learn about their benefits, acceptance rises. Education matters enormously. When rural Rwandan farmers were taught that iron beans could improve children's school performance and women's work capacity, adoption took off.
One of the most frustrating aspects of biofortification—especially for GMO varieties—is the agonizingly slow pace of regulatory approval.
Golden Rice spent over two decades navigating biosafety reviews, field trials, environmental impact assessments, and political opposition before gaining approval in the Philippines in 2021. Bangladesh approved it for cultivation in 2019. But in many countries where vitamin A deficiency is most severe, it remains unapproved.
Why so slow? Partly because GMO regulations are strict, requiring extensive testing and documentation. Partly because regulatory agencies are under-resourced and face political pressure. Partly because activist campaigns create public fear that governments feel compelled to address through caution.
The result is a tragic irony: the crops most urgently needed to save lives face the highest barriers to deployment. Meanwhile, conventionally bred biofortified crops move through the system faster, even though they may deliver lower nutrient boosts.
Some scientists and advocates argue for streamlined approval processes for biofortified crops developed by public institutions for humanitarian purposes, with safety data transparently published. Others warn against lowering safety standards, arguing that public trust depends on rigorous review.
The case for biofortification isn't just humanitarian—it's economic.
Micronutrient deficiencies cost developing economies billions annually in lost productivity, healthcare expenses, and cognitive impairment that reduces educational achievement and earning potential. Iron-deficient workers have lower physical work capacity. Zinc-deficient children have weaker immune systems and miss more school. Vitamin A-deficient populations suffer higher child mortality and maternal complications.
Biofortification addresses these costs at the source. When women in Rwanda consume iron beans and their memory, attention, and work performance improve, they earn more. When children in India eat zinc-rich pearl millet and spend less time sick, they attend school more consistently and learn more effectively.
The cost per disability-adjusted life year (DALY) saved through biofortification ranges from $15 to $20—far below the World Bank's $270 threshold for highly cost-effective interventions.
The Copenhagen Consensus estimate of $17 in benefits per dollar invested reflects this multiplier effect. Better nutrition ripples through communities: healthier workers, smarter students, fewer medical bills, more productive farms.
Farmers benefit too. Many biofortified varieties are selected not just for nutrition but for yield, disease resistance, drought tolerance, and other agronomic traits. Iron beans in Rwanda perform as well or better than conventional varieties in the field. High-zinc wheat in India and Pakistan tolerates marginal soils better than standard varieties. Farmers adopt them because they make economic sense, not just nutritional sense.
There's a darker twist to the biofortification story. As climate change accelerates, the nutrient content of crops is declining.
Elevated atmospheric CO2 levels cause crops to grow faster but dilute their nutrient concentrations. Studies show that rising CO2 reduces iron, zinc, and protein levels in wheat, rice, and other staples. Soil degradation from intensive farming and erosion further depletes micronutrients. Temperature extremes and changing rainfall patterns stress plants, reducing nutrient uptake.
The result: just as the global population grows, and just as malnutrition remains widespread, the food we're growing is becoming less nutritious.
Biofortification offers a partial solution. By breeding crops with higher baseline nutrient levels, scientists can counteract some of the CO2-driven nutrient dilution. Climate-resilient biofortified varieties—bred for both high micronutrients and tolerance to heat, drought, or flooding—become doubly valuable.
This is why organizations like HarvestPlus and partners are increasingly integrating climate adaptation into biofortification breeding programs. The goal isn't just to feed more people, but to feed them better food in a warming world.
Biofortification research is accelerating, with dozens of crops and nutrients under development or in field trials.
CRISPR gene editing is opening new possibilities, allowing precise edits to boost nutrient pathways without inserting foreign genes. Regulators in some countries treat CRISPR-edited crops more like conventionally bred varieties than GMOs, which could speed deployment.
Beyond iron, zinc, and vitamin A, scientists are working on biofortification for selenium, iodine, folate, and other micronutrients. Multi-nutrient biofortification—breeding or engineering crops to simultaneously improve several nutrients—is becoming feasible thanks to our growing understanding of nutrient correlations and metabolic pathways.
Fruits and vegetables are also being targeted. While staple grains and legumes are the priority for reaching the rural poor, biofortified fruits with enhanced vitamin C, folate, or antioxidants could benefit urban and peri-urban populations.
There's also growing interest in biofortification of animal feed crops. Biofortifying maize, sorghum, or soybeans used for livestock could boost nutrient levels in meat, milk, and eggs—potentially reaching populations who consume animal products but have limited access to fresh produce.
For all its promise, biofortification faces a critical question: Will it actually reach the poorest and most malnourished, or will it primarily benefit wealthier farmers and consumers?
The evidence so far is cautiously optimistic. Programs like HarvestPlus deliberately target smallholder farmers in low-income, nutrient-deficient regions. They partner with national agricultural research institutes and extension services to ensure biofortified seed is available and affordable. Many varieties are open-pollinated, meaning farmers can save and replant seed without purchasing new stock each season.
In Rwanda, Uganda, Mozambique, and elsewhere, adoption of biofortified crops has been highest among the rural poor who grow their own food—precisely the population most affected by hidden hunger.
Still, risks remain. If seed companies shift toward proprietary, patent-protected biofortified varieties, access for poor farmers could shrink. If marketing of biofortified foods targets urban middle-class consumers willing to pay premium prices, rural subsistence farmers may be left out. If research funding flows disproportionately toward crops consumed by wealthier populations (wheat, maize) rather than orphan crops critical to the poorest (cassava, millet, sorghum), inequity could widen.
Advocates emphasize that sustained public funding, strong intellectual property agreements that protect farmer access, and participatory research involving farming communities are essential to ensuring biofortification serves those who need it most.
Biofortification isn't humanity's first attempt to engineer better nutrition into staple foods. History offers both inspiration and caution.
In the early 20th century, iodized salt virtually eliminated goiter in developed countries. Vitamin D-fortified milk ended rickets as a major childhood disease. Folic acid fortification of flour dramatically reduced neural tube birth defects. These were public health triumphs driven by adding nutrients to processed foods.
But they required infrastructure—centralized processing, regulation, enforcement. They worked in wealthy countries with industrial food systems. They largely failed to reach the rural poor in low-income countries who couldn't afford processed foods and didn't have access to supply chains.
Biofortification learns from these successes and failures. Like fortification, it targets the foods people already eat. But unlike industrial fortification, it embeds nutrition into the crop itself, making it accessible to subsistence farmers. It's decentralized and doesn't depend on functional supply chains or enforcement.
The Green Revolution offers another lesson. In the mid-20th century, plant breeders developed high-yielding wheat and rice varieties that dramatically increased food production and prevented mass starvation in Asia and Latin America. But the Green Revolution was criticized for environmental damage, dependence on chemical inputs, and exacerbating inequality between large and small farmers.
Biofortification aims to avoid those pitfalls by prioritizing crops and breeding strategies suited to low-input, smallholder farming systems. The hope is to achieve a "nutrition revolution" without repeating the mistakes of the Green Revolution.
For most readers, biofortification feels distant—something happening in laboratories and foreign fields. But there are ways to engage and support this work.
Farmers in biofortification target regions can seek out varieties through national agricultural extension services or NGOs like HarvestPlus. Planting iron beans, vitamin A maize, or high-zinc wheat benefits not just your own family but also contributes to broader adoption and seed availability.
Consumers, especially in urban areas, can look for biofortified products when they become available in markets. In some countries, orange sweet potato, orange maize, and iron beans are starting to appear in retail channels. Choosing them signals demand and supports farmers.
Policymakers and development professionals can advocate for increased funding for biofortification research and distribution. Despite its cost-effectiveness, biofortification receives a small fraction of the funding directed toward supplementation and fortification programs. Reallocating resources toward breeding and distributing biofortified crops could yield far greater long-term impact.
Teachers and health educators can incorporate biofortification into curricula and community health programs. Explaining why orange sweet potato is healthier than white, or why iron beans make children smarter, can shift cultural preferences and accelerate adoption.
Citizens in countries where GMO regulations block beneficial biofortified crops can engage in informed debate. Understanding the science and weighing risks against benefits—especially for humanitarian crops like Golden Rice—enables more nuanced policy conversations.
Biofortification has come a long way in two decades, but major hurdles remain.
Scaling up is hard. Moving from a successful pilot project in one district to national or regional adoption requires seed production capacity, distribution networks, farmer training, and sustained funding. Many promising biofortified varieties remain confined to small areas because scaling infrastructure is missing.
Monitoring and evaluation are complex. Unlike a vaccination campaign where you can count doses administered, tracking biofortification impact requires measuring crop adoption rates, consumption patterns, and health outcomes over years. Data systems in low-income countries often aren't up to the task.
Sustainability is uncertain. Donor-funded programs like HarvestPlus have driven most progress to date. What happens when funding cycles end? Will national governments and seed companies pick up the slack? Will farmers continue to choose biofortified varieties, or revert to familiar types?
Nutrition is multifactorial. Biofortification addresses micronutrient deficiencies, but malnutrition has many causes—poverty, poor sanitation, infectious disease, inadequate healthcare, gender inequality. No single intervention solves everything. Biofortification works best as part of comprehensive development strategies that tackle root causes.
Yet the opportunities are immense. Genomic tools are advancing rapidly, making biofortification faster and more precise. International collaborations are expanding the pipeline of crops and nutrients under development. Public awareness is growing. Regulatory pathways, while still slow, are becoming better defined.
And most important, millions of farming families are already choosing to grow these crops because they work—they yield well, taste good, and make their children healthier. That organic, farmer-driven adoption is the ultimate validation.
The biofortification movement doesn't look like most technology revolutions. There are no billion-dollar IPOs, no sleek product launches, no viral marketing campaigns. There's just rice that's slightly yellower, beans that are slightly darker, sweet potatoes that are orange instead of white.
But in a world where 2 billion people suffer from hidden hunger, where children go blind from vitamin A deficiency and women lose years of productive life to anemia, where climate change is quietly sapping nutrition from our food, these small differences matter enormously.
The shift from asking "How do we get nutrients to people?" to "How do we grow nutrients in the food people already eat?" represents a fundamental reimagining of nutrition intervention. It moves upstream. It's preventive, decentralized, and sustainable. It leverages agriculture—humanity's oldest technology—to solve a problem that high-tech solutions have struggled with for decades.
We're watching the early stages of what could become a genuine revolution in global health. Not because a new drug or device was invented, but because scientists, farmers, and communities are quietly transforming the crops we've eaten for millennia to carry the nutrients our bodies desperately need.
The question isn't whether biofortification can work—evidence shows it does. The question is whether we'll invest in it, scale it, and deploy it fast enough to reach the billions who need it before another generation grows up stunted, weakened, and limited by deficiencies we have the power to prevent.
History may look back at biofortification as the moment we finally cracked hidden hunger. Or it may ask why, with all the tools and knowledge at hand, we waited so long to act.
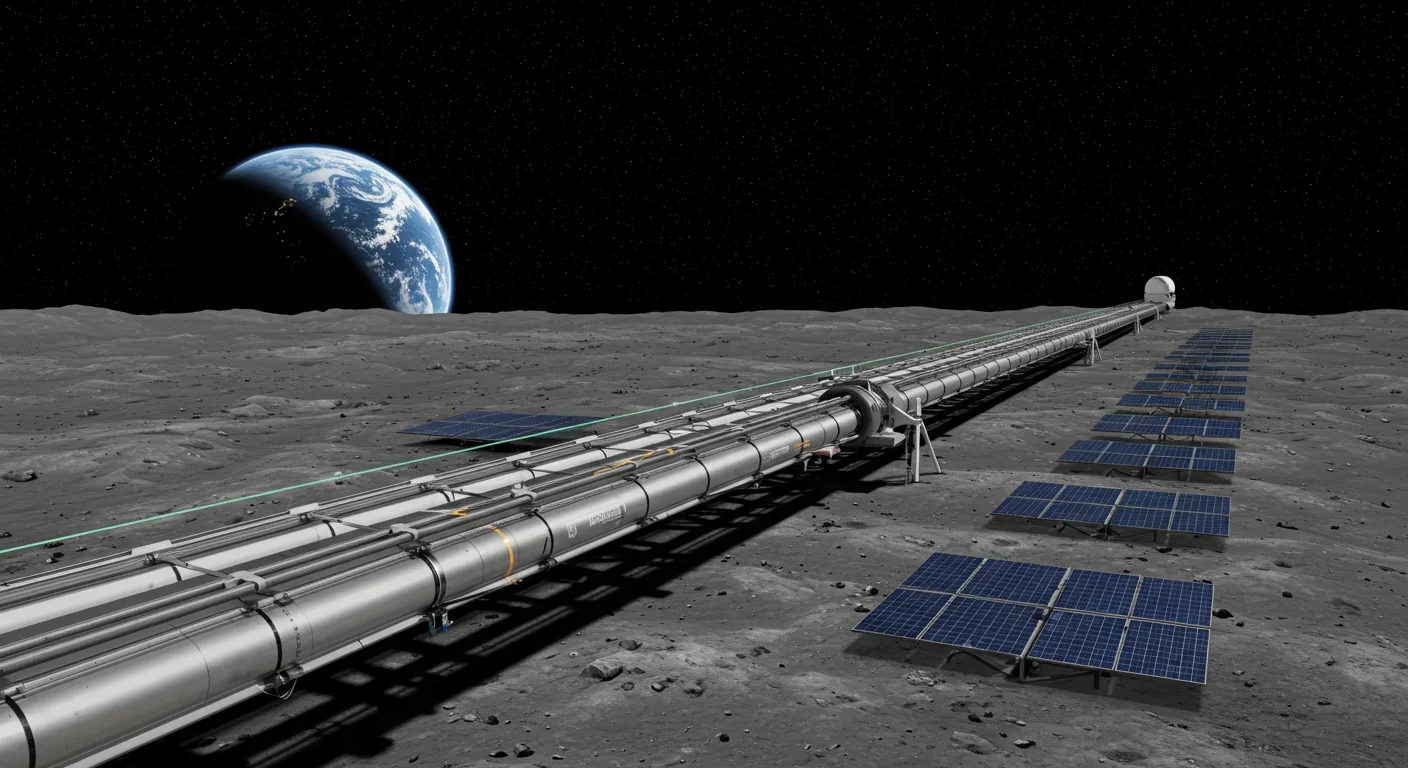
Lunar mass drivers—electromagnetic catapults that launch cargo from the Moon without fuel—could slash space transportation costs from thousands to under $100 per kilogram. This technology would enable affordable space construction, fuel depots, and deep space missions using lunar materials, potentially operational by the 2040s.

Ancient microorganisms called archaea inhabit your gut and perform unique metabolic functions that bacteria cannot, including methane production that enhances nutrient extraction. These primordial partners may influence longevity and offer new therapeutic targets.

CAES stores excess renewable energy by compressing air in underground caverns, then releases it through turbines during peak demand. New advanced adiabatic systems achieve 70%+ efficiency, making this decades-old technology suddenly competitive for long-duration grid storage.

Human children evolved to be raised by multiple caregivers—grandparents, siblings, and community members—not just two parents. Research shows alloparenting reduces parental burnout, improves child development, and is the biological norm across cultures.

Soft corals have weaponized their symbiotic algae to produce potent chemical defenses, creating compounds with revolutionary pharmaceutical potential while reshaping our understanding of marine ecosystems facing climate change.

Generation Z is the first cohort to come of age amid a polycrisis - interconnected global failures spanning climate, economy, democracy, and health. This cascading reality is fundamentally reshaping how young people think, plan their lives, and organize for change.

Zero-trust security eliminates implicit network trust by requiring continuous verification of every access request. Organizations are rapidly adopting this architecture to address cloud computing, remote work, and sophisticated threats that rendered perimeter defenses obsolete.