Bombardier Beetle Chemical Defense: Nature's Micro Engine

TL;DR: Bdelloid rotifers have survived 80 million years without sex—an evolutionary impossibility that's rewriting biology. These microscopic animals compensate through gene theft from bacteria, transgenerational DNA repair, and using desiccation to eliminate parasites. Their survival toolkit is inspiring breakthroughs in vaccine preservation, space travel, and antibiotic development. They prove asexual life can thrive indefinitely with the right workarounds—challenging our assumptions about evolution's rules.

By 2030, scientists predict that breakthroughs in understanding asexual reproduction could revolutionize how we preserve cells, combat disease, and even approach human longevity. At the center of this revolution? Microscopic animals no bigger than a grain of sand that have been breaking biology's most fundamental rule for 80 million years.
Bdelloid rotifers are evolutionary rebels. While nearly every complex organism on Earth depends on sexual reproduction to shuffle genes and fend off extinction, these tiny freshwater creatures have thrived for tens of millions of years without a single male in sight. They've survived being frozen in permafrost for 24,000 years, blasted with radiation doses that would liquefy human tissue, and dried out so completely that their bodies crack into dust—only to spring back to life when water returns.
What researchers are discovering about how bdelloids accomplish this feat is rewriting the rulebook on evolution, DNA repair, and the very necessity of sex itself. Their strategies—from stealing genes from bacteria to repairing shattered chromosomes across generations—are inspiring new approaches to everything from crop engineering to space colonization. This is the story of nature's greatest contrarians, and what their 80-million-year experiment reveals about life's hidden possibilities.
In 1702, Dutch lens-maker Antonie van Leeuwenhoek first observed bdelloid rotifers under his primitive microscope, marveling at these "wheel animals" whose crown of beating cilia resembled spinning wheels. For the next three centuries, biologists catalogued over 450 species across freshwater habitats worldwide—from Antarctic meltwater pools to the ephemeral puddles atop mossy rocks. But one observation grew increasingly puzzling: no one, across centuries of study, had ever found a male bdelloid.
This wasn't just unusual. It was theoretically impossible.
Evolutionary biology rests on a fundamental premise: sexual reproduction, despite its enormous costs, is essential for long-term survival. Sex requires organisms to invest energy finding mates, sacrifice half their genetic contribution to each offspring (the infamous "two-fold cost"), and risk exposure to predators and disease during mating. Yet nearly all complex life pays these costs because sex provides an irreplaceable benefit—genetic recombination.
When chromosomes pair during meiosis, they swap segments in a process called crossing over, shuffling parental genes into novel combinations. This genetic reshuffling serves two critical functions. First, it helps repair DNA damage through homologous recombination, using an intact chromosome copy as a template. Second, it generates genetic diversity that allows populations to outpace evolving parasites and adapt to changing environments. The Red Queen hypothesis, named for the character in Through the Looking-Glass who must run constantly just to stay in place, predicts that without this genetic shuffling, asexual lineages should spiral into extinction within thousands of generations as parasites adapt and deleterious mutations accumulate.
Bdelloid rotifers have persisted for approximately 80 million years—roughly 100 million generations—without meiosis, without recombination, without males, without any observed sexual reproduction whatsoever. Evolutionary biologist John Maynard Smith famously called them an "evolutionary scandal." They shouldn't exist. Yet they do—and they're thriving.
Bdelloid rotifers don't just defy evolutionary theory; they routinely survive conditions that would annihilate most multicellular life. Their resilience reads like science fiction.
In 2021, Russian researchers extracted sediment core samples from 24,000-year-old Siberian permafrost. When the ancient ice thawed, bdelloid rotifers—frozen since the late Pleistocene, when woolly mammoths still roamed—revived and began reproducing within hours. No resting eggs. No specialized dormancy stage. Just ordinary individuals who had been interrupted mid-life, pressed pause for 240 centuries, and resumed as if awakening from an afternoon nap.
Radiation resistance borders on the supernatural. While 5-10 Gray of ionizing radiation proves lethal to humans, bdelloid rotifers shrug off doses exceeding 1,000 Gray—comparable to the radiation-resistant bacterium Deinococcus radiodurans. Researchers have observed bdelloid genomes shattered into hundreds of fragments by radiation, yet the animals repair every break and reproduce normally with only a 20% reduction in fertility.
Their signature superpower, however, is anhydrobiosis—life without water. When their freshwater pools evaporate, bdelloids contract into a dehydrated structure called a "tun," losing up to 99% of their body water. In this state, with metabolism reduced to undetectable levels, they can survive vacuum conditions, temperatures from -196°C to 151°C, and years of complete desiccation. Rehydration reverses the process in hours, with no apparent damage.
This extreme resilience isn't a quirk—it's central to understanding how bdelloids have sidestepped sex's necessity. Because their survival strategy holds the key to their genetic innovation.
When researchers sequenced the first bdelloid genome in 2013, they discovered something astounding: approximately 8-10% of bdelloid genes had been stolen from other organisms—bacteria, fungi, plants, even viruses. This proportion represents the highest level of horizontal gene transfer (HGT) ever documented in any animal.
Horizontal gene transfer—acquiring DNA from unrelated species rather than inheriting it from parents—is common in bacteria, where genes conferring antibiotic resistance can spread through entire microbial communities. In animals, however, HGT is extraordinarily rare because germ cells (eggs and sperm) are typically segregated and protected. Bdelloids have inadvertently turned their greatest vulnerability—their fragility during desiccation—into an evolutionary advantage.
Here's how the heist works. As bdelloids dry out, their DNA doesn't just accumulate damage—it shatters into pieces. Their cell membranes become permeable, and the barrier between self and environment breaks down. Bdelloids feed on bacteria, algae, and organic detritus; when desiccation scrambles cellular integrity, fragments of DNA from recently consumed prey become accessible. During rehydration, as the bdelloid's repair machinery frantically reassembles its genome, foreign DNA fragments can integrate into chromosomes.
Most HGT events are probably neutral or harmful, but over millions of years, the rare beneficial acquisitions accumulate. Bdelloid genomes now harbor bacterial genes encoding antibiotic synthesis, antioxidant enzymes, and metabolic pathways absent in other animals. A 2024 study demonstrated that bdelloids infected with fungal parasites dramatically upregulate bacterial-derived genes producing antimicrobial peptides—stolen weaponry repurposed against new threats.
In 2022, researchers discovered something even more remarkable: a bdelloid-specific epigenetic system, captured from bacteria approximately 60 million ago, that modifies DNA in ways previously unknown in animals. The bacterial methyltransferase gene produces N4-methylcytosine, a chemical mark that helps suppress transposable elements ("jumping genes" that can wreak havoc by inserting themselves randomly throughout the genome). This borrowed regulatory system provides an extra layer of genome defense that sexual organisms lack—a bacterial Band-Aid applied to an asexual wound.
Horizontal gene transfer effectively replaces one function of sexual recombination: introducing genetic novelty. While sexual organisms generate diversity by shuffling existing genes, bdelloids import entirely new genes from across the tree of life. It's not gene shuffling—it's gene piracy. And it works.
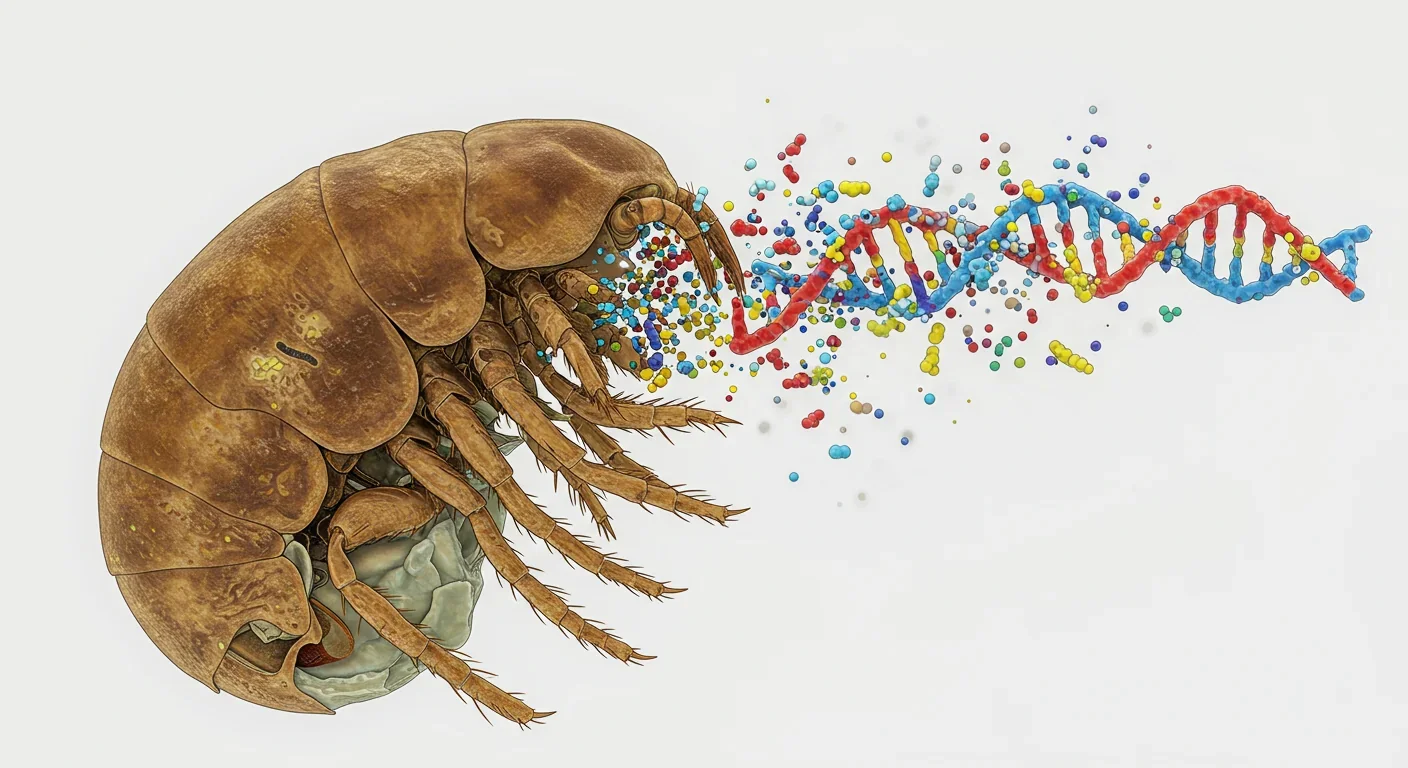
But importing bacterial genes doesn't solve the second critical function of sex: efficient DNA repair through homologous recombination. Sexual organisms use meiosis to pair up homologous chromosomes, allowing accurate repair of double-strand breaks—the most dangerous form of DNA damage—by copying information from an intact partner chromosome. Asexual bdelloids lack meiosis. So how do they fix hundreds of radiation-induced breaks without templates?
The answer emerged in 2024 from a team studying the bdelloid species Adineta vaga. Researchers discovered a novel repair mechanism they termed BIHER: Break-Induced Homologous Extension Repair. Unlike conventional homologous recombination, which requires two chromosome ends and intimate pairing, BIHER operates on single free DNA ends, extending them across generations to repair fragmentation.
Here's what makes BIHER revolutionary: it's transgenerational. When a bdelloid's genome shatters during desiccation or radiation exposure, BIHER doesn't attempt immediate, complete repair. Instead, repair machinery extends DNA from broken ends during replication, gradually rebuilding chromosomes as the organism reproduces. Daughters inherit partially repaired genomes, and repair continues incrementally across multiple generations. Genomic analysis of irradiated bdelloid lineages reveals the molecular signature of BIHER—extended homologous sequences and single-end crossover events absent in sexual species.
This strategy is enabled by bdelloids' unusual chromosome architecture. Unlike most animals, whose chromosomes have a single centromere (the attachment point for cellular machinery that pulls chromosomes apart during division), bdelloid chromosomes are holocentric—their centromeric activity is distributed along the entire length. This holocentric structure creates multiple independent attachment sites, allowing fragmented chromosomes to segregate properly during cell division even while undergoing repair. It's like having multiple handles on a suitcase; if one breaks, others keep it functional.
The same team discovered that bdelloids retain meiotic recombination genes—they just repurposed them. Instead of using these genes during sexual reproduction (which never happens), bdelloids deploy meiotic machinery during egg development for DNA repair and to generate limited genetic variation through gene conversion, a process that copies small DNA segments between similar sequences. This gene conversion during mitosis helps purge deleterious mutations without requiring a sexual partner—a solo performance of a duet.
BIHER and gene conversion together provide a makeshift substitute for meiotic recombination. It's not as efficient as sex—the organism accumulates more mutations over time—but combined with horizontal gene transfer's influx of fresh DNA, it's enough. Barely. But for 80 million years, barely has been sufficient.
Even if bdelloids can repair DNA and import new genes, the Red Queen hypothesis poses a brutal challenge: parasites. Coevolving pathogens track host genotypes, adapting to exploit common genetic combinations. Sexual reproduction generates rare, unpredictable genotypes that parasites haven't yet adapted to, providing an evolutionary escape hatch. Asexual clones, in contrast, should be sitting ducks—genetically identical targets for specialized parasites.
So where are the bdelloid-specific parasites?
A clever 2010 study by Wilson and Sherman provided the answer. They demonstrated that bdelloid rotifers face infection from generalist fungal parasites, but desiccation—the same process that enables horizontal gene transfer—also serves as a sterilization protocol. When bdelloids dry out, fungal infections die. The rotifer survives; the parasite doesn't.
Moreover, desiccated bdelloids are small enough to be carried by wind currents across vast distances. When they rehydrate in a new pool hundreds of kilometers away, they colonize parasite-free environments. By the time local parasites adapt, another desiccation cycle may disperse the population again. Bdelloids have weaponized their own fragility, turning environmental instability into an immune system.
This strategy works because bdelloids inhabit ephemeral habitats—temporary puddles, water films on mosses, transient meltwater. Constant environmental disruption prevents parasites from establishing stable, coevolving relationships. Bdelloids essentially live in evolutionary refugee camps, always moving before enemies can catch up. It's not a perfect solution—recent studies have documented fungal infections in bdelloid populations—but it buys enough time for other mechanisms to compensate.
In sexual organisms, each gene exists in two copies (alleles)—one from each parent. These alleles are similar but not identical, providing genetic redundancy and the raw material for natural selection. Bdelloid rotifers, reproducing asexually, should have identical allele copies. Over time, without recombination to shuffle alleles between lineages, all bdelloids of a species should converge toward genetic uniformity.
But they haven't.
Bdelloid genomes contain two or more divergent copies of every gene, and these copies have diverged far more than expected—in some cases, the sequence differences suggest they've been evolving independently for tens of millions of years. A 2007 study of the gene lea (Late Embryogenesis Abundant) in Adineta ricciae revealed that the two copies, once functionally identical, have diverged to perform distinct roles: one protects proteins from aggregation during desiccation, the other stabilizes cell membranes.
This is functional divergence—when duplicated genes evolve complementary, specialized functions. In sexual organisms, recombination constantly re-mixes alleles, preventing such divergence. In asexual bdelloids, lacking recombination, duplicate genes are free to drift apart and experiment with new functions. It's an evolutionary strategy unique to asexuality, turning a liability into an asset.
Think of it as evolutionary jazz improvisation. Sexual organisms play the same song repeatedly, swapping musicians but keeping the arrangement. Bdelloids let each musician riff independently, creating harmonies that would be impossible in the original composition. Over millions of years, this produces a repertoire of specialized gene functions unavailable to sexual species.
A 2013 genomic analysis revealed another peculiarity: bdelloid alleles are not arranged in the paired, homologous chromosomes typical of sexual organisms. Instead, they're scattered in complex, intrachromosomal repeats—a tangled genomic architecture that likely makes meiotic pairing mechanically impossible. Bdelloids have been asexual so long, their genomes have reorganized in ways incompatible with sex. They've burned the evolutionary bridges behind them.
In 2008, however, a bombshell study by Matthew Meselson and colleagues reported evidence of meiotic activity in bdelloid rotifers—a finding that seemed to contradict centuries of observation. The team identified meiotic gene expression and chromosome behavior during egg development that resembled meiosis, albeit highly unusual.
This "meiosis" doesn't produce haploid cells or facilitate fertilization—there are still no males, no sperm, no genetic exchange between individuals. Instead, bdelloids appear to undergo a non-reductional meiotic process that restores heterozygosity (having two different alleles) without reducing chromosome number. The biological function remains unclear, but researchers speculate it may facilitate gene conversion or chromosome repair.
Does this discovery mean bdelloids aren't truly asexual? Not quite. They're not engaging in sexual reproduction as biologists define it—genetic exchange between individuals—but they've retained molecular machinery associated with meiosis and repurposed it for solo genome maintenance. It's like keeping a car engine to power a generator; the function has changed, but the hardware remains.
This finding complicates the narrative but doesn't undermine it. Bdelloids still lack males, still reproduce clonally, still haven't been observed exchanging genes between individuals for 80 million years. What they do have is a cobbled-together toolkit of repurposed meiotic genes, horizontal gene transfer, desiccation-mediated parasite avoidance, and transgenerational repair mechanisms—a Rube Goldberg machine of evolutionary workarounds that, collectively, substitute for sex.
The bdelloid survival toolkit has captured attention far beyond evolutionary biology. In 2023, researchers aboard the International Space Station subjected Adineta vaga to 12 days of microgravity and space radiation. Transcriptomic analysis revealed upregulation of DNA repair pathways, protein synthesis machinery, and—significantly—horizontal gene transfers. Even in the harshness of space, bdelloids maintained genomic stability, reproduced successfully, and exhibited adaptive gene expression.
This resilience has profound implications for space colonization. Long-duration spaceflight exposes astronauts to cosmic radiation and microgravity-induced cellular stress. Understanding how bdelloids tolerate such conditions for generations could inform countermeasures for human space travel—pharmacological interventions that enhance DNA repair, cell preservation techniques inspired by anhydrobiosis, or genetic modifications based on horizontally transferred protective genes.
Biotechnology companies are already exploring bdelloid-inspired preservation strategies. Anhydrobiosis offers a blueprint for stabilizing vaccines, biologics, and cell therapies without refrigeration—a breakthrough for global health in regions lacking cold-chain infrastructure. Researchers are investigating the intrinsically disordered proteins bdelloids use during desiccation, which prevent protein aggregation and membrane damage, as templates for stabilizing enzymes used in industrial processes.
The bacterial-derived methyltransferase system that bdelloids captured 60 million years ago has also sparked interest as a potential genome-editing tool. Just as CRISPR-Cas9 was adapted from bacterial immune systems, this novel epigenetic mark could enable new methods for controlling transposable elements or regulating gene expression in crops and livestock.
Bdelloid-derived antimicrobial peptides are under investigation as alternatives to conventional antibiotics. Unlike antibiotics that target specific bacterial pathways (and thus foster resistance), the non-ribosomal peptides bdelloids synthesize using stolen bacterial genes may offer broader-spectrum activity with lower toxicity. In an era of rising antibiotic resistance, these ancient weapons might become tomorrow's medicines.

The bdelloid story forces a reckoning with some of biology's most cherished assumptions. For decades, the ubiquity of sexual reproduction seemed to prove its necessity. Asexuality was viewed as an evolutionary dead-end, viable only in the short term. Bdelloids shatter that narrative.
They demonstrate that asexual lineages can persist indefinitely—if they compensate through alternative mechanisms. Horizontal gene transfer can substitute for genetic recombination's novelty. Transgenerational DNA repair can replace homologous recombination's precision. Environmental instability can substitute for genotypic diversity in evading parasites. Functional divergence of gene copies can generate adaptive complexity without allelic shuffling.
None of these mechanisms is as efficient as sex. Bdelloids accumulate more deleterious mutations, repair DNA more slowly, and adapt less readily to stable environments than their sexual relatives. But in the ephemeral, radiation-exposed, desiccation-prone habitats they occupy, the trade-offs favor their patchwork strategy. Evolution doesn't demand perfection—only adequacy.
Bdelloids also illuminate the true cost of sex. If asexual organisms can survive 80 million years using workarounds, why do most complex organisms bother with sex? The answer may be environmental. Bdelloids thrive precisely because their habitats are unstable. Constant desiccation cycles and dispersal prevent parasite specialization. In stable environments—rainforest ponds, deep lakes, oceanic ecosystems—parasites have time to adapt, and sexual reproduction's Red Queen advantage becomes decisive. Sex isn't universally necessary; it's necessary in most environments most organisms inhabit.
For humans, this offers a humbling lesson. We've long viewed sexual reproduction as a biological imperative, a defining feature of complex life. Bdelloids remind us that biology is more flexible, more inventive, and more contingent than we assume. The "rules" of evolution are really probabilities, and outliers reveal possibilities the mainstream never explores.
Could other multicellular organisms adopt bdelloid-like strategies? Probably not easily. Bdelloids evolved their toolkit over tens of millions of years, in specific ecological niches, with body plans and life cycles that facilitate desiccation and horizontal gene transfer. Replicating this in, say, a mammal would require radical reorganization of development, reproduction, and genome architecture—changes so extensive they'd produce something unrecognizable.
But bdelloid mechanisms could be borrowed piecemeal. Enhancing DNA repair using BIHER-inspired approaches. Engineering crops to acquire stress-tolerance genes horizontally from bacteria. Designing preservation protocols based on anhydrobiosis. Bdelloids won't make sex obsolete, but their solutions may help solve problems sex never addressed.
As climate change accelerates, ephemeral habitats—temporary pools, glacial meltwater, seasonal wetlands—are expanding in some regions and vanishing in others. Bdelloid rotifers, masters of the transient, may become model organisms for understanding adaptation to environmental instability. Their strategies for surviving unpredictable conditions could inform conservation efforts for species facing increasingly volatile climates.
Recent discoveries continue to upend assumptions. In 2024, researchers identified convergent evolution of desiccation-tolerance genes between bdelloid and monogonont rotifers (which reproduce both sexually and asexually), suggesting that anhydrobiosis adaptations may be more widespread and transferable than thought. Heat-shock proteins and antioxidant enzymes—the molecular machinery of survival—appear in distantly related organisms facing similar challenges, hinting at a conserved toolkit for extremophile lifestyles.
Work is underway to determine whether bdelloids engage in cryptic sexual events—rare, unobserved episodes of genetic exchange that might supplement their asexual strategy. If such events occur even once every few thousand generations, they could dramatically alter our understanding of their evolutionary persistence. The possibility of "mostly asexual" rather than "strictly asexual" reproduction would reframe bdelloids not as evolutionary rule-breakers, but as organisms exploiting a hybrid strategy invisible to conventional observation.
What remains certain is that bdelloid rotifers have survived longer without sex than any other complex animal—and they're still going. Every drop of pond water, every splash of melted snow, every microscopic water film on a forest leaf potentially harbors these tiny time-travelers, carrying genomes shaped by 80 million years of evolutionary improvisation. They're living proof that nature's creativity exceeds our theories, that the "impossible" often just means "we haven't figured it out yet."
By 2030, as researchers unravel more of the bdelloid toolkit, we may find ourselves applying lessons from these microscopic rebels to challenges from space travel to agriculture to medicine. The organisms that broke biology's rules might just teach us how to rewrite them—not to eliminate sex, but to understand its true role and recognize the hidden alternatives life has been experimenting with all along.
The bdelloid rotifers have been whispering a secret for 80 million years: evolution is not a rigid script but an open-ended conversation, full of unexpected solutions to ancient problems. We're only now learning to listen.

Recent breakthroughs in fusion technology—including 351,000-gauss magnetic fields, AI-driven plasma diagnostics, and net energy gain at the National Ignition Facility—are transforming fusion propulsion from science fiction to engineering frontier. Scientists now have a realistic pathway to accelerate spacecraft to 10% of light speed, enabling a 43-year journey to Alpha Centauri. While challenges remain in miniaturization, neutron management, and sustained operation, the physics barriers have ...

Epigenetic clocks measure DNA methylation patterns to calculate biological age, which predicts disease risk up to 30 years before symptoms appear. Landmark studies show that accelerated epigenetic aging forecasts cardiovascular disease, diabetes, and neurodegeneration with remarkable accuracy. Lifestyle interventions—Mediterranean diet, structured exercise, quality sleep, stress management—can measurably reverse biological aging, reducing epigenetic age by 1-2 years within months. Commercial ...

Data centers consumed 415 terawatt-hours of electricity in 2024 and will nearly double that by 2030, driven by AI's insatiable energy appetite. Despite tech giants' renewable pledges, actual emissions are up to 662% higher than reported due to accounting loopholes. A digital pollution tax—similar to Europe's carbon border tariff—could finally force the industry to invest in efficiency technologies like liquid cooling, waste heat recovery, and time-matched renewable power, transforming volunta...

Humans are hardwired to see invisible agents—gods, ghosts, conspiracies—thanks to the Hyperactive Agency Detection Device (HADD), an evolutionary survival mechanism that favored false alarms over fatal misses. This cognitive bias, rooted in brain regions like the temporoparietal junction and medial prefrontal cortex, generates religious beliefs, animistic worldviews, and conspiracy theories across all cultures. Understanding HADD doesn't eliminate belief, but it helps us recognize when our pa...

The bombardier beetle has perfected a chemical defense system that human engineers are still trying to replicate: a two-chamber micro-combustion engine that mixes hydroquinone and hydrogen peroxide to create explosive 100°C sprays at up to 500 pulses per second, aimed with 270-degree precision. This tiny insect's biochemical marvel is inspiring revolutionary technologies in aerospace propulsion, pharmaceutical delivery, and fire suppression. By 2030, beetle-inspired systems could position sat...

The U.S. faces a catastrophic care worker shortage driven by poverty-level wages, overwhelming burnout, and systemic undervaluation. With 99% of nursing homes hiring and 9.7 million openings projected by 2034, the crisis threatens patient safety, family stability, and economic productivity. Evidence-based solutions—wage reforms, streamlined training, technology integration, and policy enforcement—exist and work, but require sustained political will and cultural recognition that caregiving is ...

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.