Bombardier Beetle Chemical Defense: Nature's Micro Engine

TL;DR: Arctic fish survive in subzero seas using antifreeze proteins that bind to ice crystals, creating a 1-2°C thermal gap between freezing and melting. These proteins evolved independently at least four times across fish families, representing one of nature's most elegant solutions to extreme cold. Scientists are now using AFP-inspired biomimetic peptoids to preserve human organs for up to 120 hours (versus 24 hours conventionally), engineer frost-resistant crops to withstand climate disruption, and improve frozen food quality. Yet climate change threatens the very species whose evolutionary innovations could save human lives—a race between technological application and ecological extinction.

In the frozen waters off Greenland, where icebergs calve into seas cold enough to kill most life in minutes, scientists recently discovered something extraordinary: a translucent fish no bigger than your thumb, glowing faintly green under their lights, swimming contentedly at temperatures that should have turned its blood to slush. When researchers analyzed its tissues, they found antifreeze protein levels higher than any fish ever recorded—a biological achievement that's now revolutionizing how we preserve human organs, protect crops from climate chaos, and reimagine what's possible when nature's oldest solutions meet our newest problems.
This isn't just another curiosity from the deep. The molecular machinery keeping Arctic fish alive in water colder than your freezer is about to change transplant medicine, rewrite agricultural genetics, and challenge everything we thought we knew about the physics of ice.
When biochemist Arthur DeVries lowered a thermometer into Antarctic waters in the late 1960s, he recorded temperatures of -1.9°C—cold enough to freeze the blood of any known vertebrate. Yet all around him, fish swam normally, seemingly oblivious to laws of thermodynamics that should have killed them. DeVries's discovery of antifreeze glycoproteins in their blood serum launched a scientific revolution that continues to accelerate today.
What makes these proteins remarkable isn't that they lower water's freezing point—common salt does that. Instead, antifreeze proteins (AFPs) perform a far more sophisticated trick: they bind directly to nascent ice crystals, forcing them to develop curved surfaces that require dramatically colder temperatures to grow. This creates a phenomenon called thermal hysteresis—a gap of 1-2°C between the temperature at which water freezes and the temperature at which existing ice melts. For fish living in -2°C seawater (the freezing point of salt water), this gap is literally the difference between life and death.
But here's where the physics gets weird. Recent research using terahertz spectroscopy revealed that AFPs don't just bind to ice surfaces—they create an "extended dynamical hydration shell," organizing water molecules up to several nanometers away into patterns that suppress ice nucleation. "We could see that the protein has an especially long-range effect on the water molecules around it," explains researcher Konrad Meister. This discovery overturned decades of assumptions about how antifreeze proteins work, revealing that their power comes not from direct ice contact but from reshaping the quantum behavior of surrounding water.
Even stranger: these proteins don't just prevent freezing—they also prevent melting. When researchers warmed Antarctic fish above the temperature at which ice should melt, internal ice crystals remained solid, locked in place by the same proteins that prevented their growth. This "anti-melt" property creates what may be the first example of ice superheating observed in nature—a phenomenon previously seen only in laboratory conditions. Some Antarctic fish may carry internal ice crystals for their entire lives, a biological compromise that enabled survival but might exact hidden physiological costs.
The story of antifreeze proteins is one of convergent evolution on a breathtaking scale. As Earth's climate cooled during the Cenozoic era—beginning around 35 million years ago when Antarctica froze and intensifying 2-3 million years ago when Arctic ice sheets formed—fish in polar waters faced an existential crisis. Those that couldn't adapt went extinct. Those that survived did so by independently inventing the same molecular solution, over and over.
Scientists have now identified at least four distinct types of AFPs (Types I-IV) plus antifreeze glycoproteins (AFGPs), each with radically different structures but converging on the same functional outcome. Type I AFPs are alanine-rich α-helices found in winter flounder and sculpins. Type II AFPs form lectin-like folds in sea ravens and smelt. Type III AFPs adopt compact globular structures in Antarctic eelpouts. Type IV AFPs have novel architectures in longhorn sculpins. AFGPs consist of repeating glycotripeptide units in Antarctic notothenioids and Arctic cod.
What's remarkable is that these proteins evolved from completely unrelated genetic starting points. Recent genomic studies traced the origin of Type I AFPs to at least four separate evolutionary events. In winter flounder, AFPs descended from a digestive enzyme. In cunner fish, they arose from duplicated GIMAP immune genes with their GTPase domains deleted. In snailfish, they emerged from transposon-derived sequences—essentially viral DNA fragments that got repurposed. In Arctic cod, AFGPs evolved de novo from non-coding DNA through a series of duplications that occurred only 2-3 million years ago—an evolutionary eyeblink.
This pattern reveals something profound about biological innovation: when selective pressure is intense enough, evolution finds the same solution through radically different paths. The constraints of ice physics leave only a narrow design space for proteins that can bind to ice crystals—specifically, they need hydrophobic flat surfaces with hydroxyl groups spaced to match the ice lattice. Nature discovered this solution four times in fish alone, and separately in insects, plants, fungi, and bacteria.
But evolution didn't stop at inventing AFPs once. Antarctic notothenioids possess 3-to-300 times more copies of genes encoding AFPs and other stress-response proteins than their warm-water relatives. Gene duplication followed by divergence allowed these fish to flood their tissues with antifreeze proteins at concentrations high enough—up to 35 mg/mL in some species—to protect against the most extreme conditions. In one recent study of zoarcoid fishes, AFP copy numbers ranged from zero to 42 functional copies across species, with the highest numbers found in the coldest, deepest habitats.
And sometimes evolution took shortcuts. In at least one documented case, smelt fish didn't evolve their own AFPs—they acquired a functional AFP gene directly from herring through horizontal gene transfer, a phenomenon more commonly associated with bacteria than complex vertebrates. This bacterial-style gene swapping between fish species suggests that when survival is at stake, evolution will exploit every available mechanism, from gradual mutation to wholesale genetic plagiarism.
To grasp how antifreeze proteins work, imagine ice not as a static solid but as a dynamic crystal constantly adding and shedding water molecules at its surface. At temperatures near the freezing point, these processes balance: ice neither grows nor shrinks. But introduce an AFP, and everything changes.
AFPs work through a mechanism called adsorption-inhibition. The protein's ice-binding surface—typically a flat array of threonine residues with precisely spaced hydroxyl groups—matches the geometric pattern of water molecules in ice. When an AFP encounters a growing ice crystal, it irreversibly binds to specific crystal planes, particularly the a-axis and c-axis faces. Once bound, the protein creates a curved ice front between adjacent binding sites. Growing ice over this curve requires adding water molecules to highly unfavorable positions, increasing surface free energy and demanding much colder temperatures for the crystal to advance.
The result is thermal hysteresis: a fish swimming at -1.9°C with AFPs in its blood won't freeze even though pure water would form ice at 0°C and even seawater would freeze at -2°C. The AFPs have created a kinetic barrier, not a thermodynamic one—the ice wants to grow, but it physically can't until temperatures drop further.

But the mechanism is even more sophisticated than simple surface binding. Using terahertz spectroscopy and molecular dynamics simulations, researchers discovered that AFPs organize water molecules up to several nanometers away into clathrate-like structures—cage formations that resist converting into ice. This "extended dynamical hydration shell" means AFPs control ice formation at a distance, through long-range restructuring of water's hydrogen-bond network.
Different AFP types employ variations on this theme. Type I AFPs are simple α-helices with hydrophobic residues (alanine, leucine) on one face creating a flat ice-binding surface. Type III AFPs use a compact β-sandwich fold with a flat ice-binding plane formed by surface loops. Insect AFPs form β-helical solenoids with repeating threonine-X-threonine (TXT) motifs that create a "threonine ladder"—two parallel rows of hydroxyl groups matching ice's lattice with extraordinary precision. These insect AFPs can be 10-100 times more potent than fish AFPs, achieving the same thermal hysteresis at far lower concentrations.
Recent computational studies revealed how subtle changes in protein scaffold geometry tune ice-binding efficiency. By varying the volume of inward-facing amino acids in β-solenoid structures, researchers can adjust the spacing of the threonine ladder, bringing it into or out of register with the ice lattice. Larger inward residues produce less concave surfaces with hydroxyl spacing closer to ice's natural geometry, enhancing binding. This insight is now guiding rational design of synthetic AFPs with optimized architectures.
One newly discovered AFP from winter flounder illustrates nature's ingenuity. This "hyperactive" protein loses all antifreeze activity at room temperature and low pH—which explains why it evaded detection for 30 years. Its activity depends on maintaining a precise three-dimensional structure stabilized by an intramolecular disulfide bond. At physiological cold temperatures and pH, it binds irreversibly to ice and generates 0.4°C more thermal hysteresis than previously known fish AFPs, enough to protect flounder down to the full -1.9°C freezing point of seawater.
Even more surprising: the conventional wisdom that AFPs require threonine's hydroxyl groups for ice binding turns out to be incomplete. When researchers mutated all threonines to valine in winter flounder AFP—replacing hydroxyl groups with purely hydrophobic methyl groups—the protein retained 94% of its antifreeze activity (1.6°C vs. 1.7°C thermal hysteresis). The hydrophobic surface and α-helical geometry proved more important than specific hydrogen bonding, suggesting that van der Waals interactions and matching the ice lattice's hydrophobic pockets drive binding as much as hydrogen bonds.
The discovery of AFPs immediately sparked questions: if these proteins can keep fish alive in frozen seas, what else might they preserve? The answers are transforming industries from medicine to agriculture to food science.
Transplant Medicine's Holy Grail
Every year, thousands of donated organs go unused because they deteriorate too quickly in conventional ice storage. Hearts remain viable for just 4-6 hours, kidneys for 24-30 hours. This narrow window forces frantic logistics and geographic constraints that leave patients dying on waiting lists while viable organs expire.
Antifreeze proteins promised a solution: preserve organs at subzero temperatures where metabolism stops entirely, without the ice crystal formation that shreds cellular membranes. But natural AFPs proved difficult to produce in quantity and didn't quite deliver the protection needed.
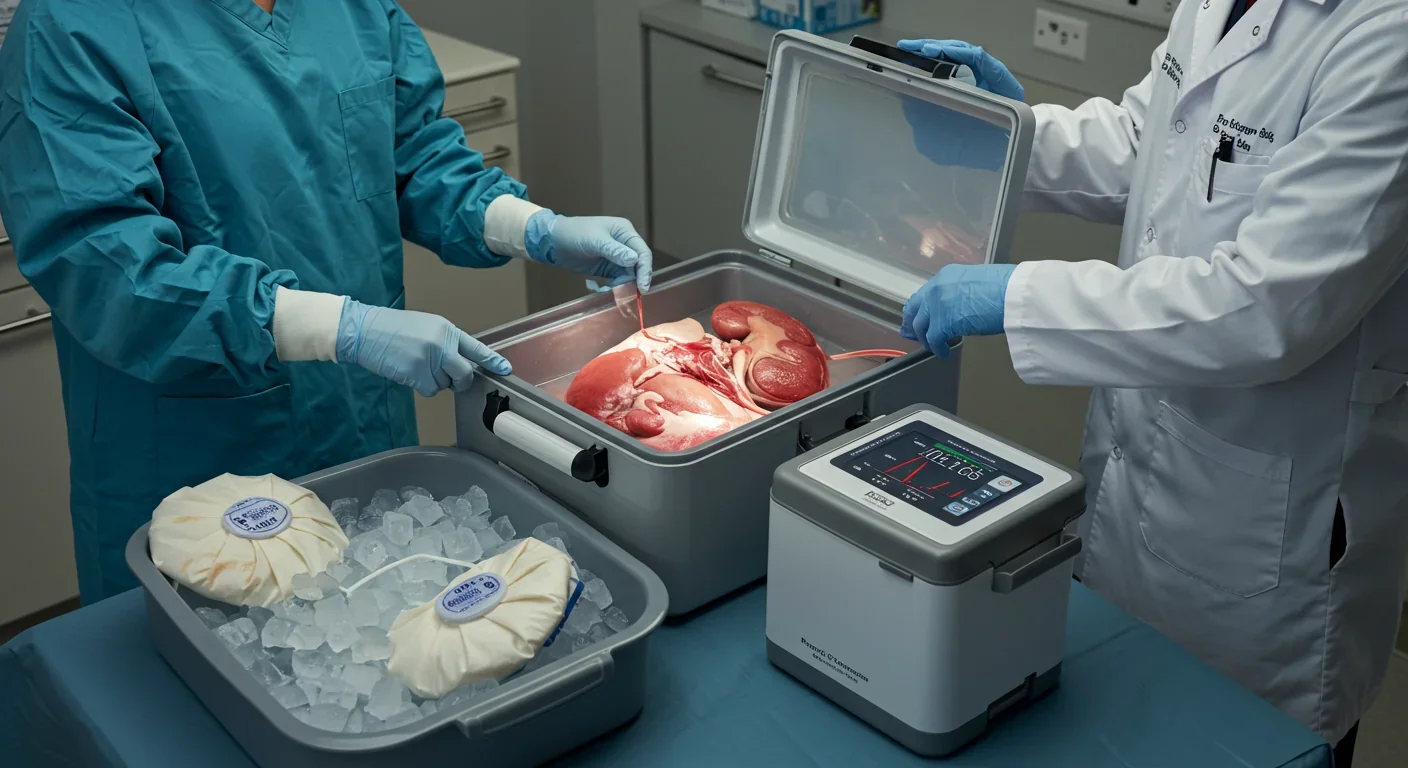
Enter X-Therma, a biotech company that took inspiration from fish AFPs but created something better: synthetic peptoids—polymer chains that mimic AFP structure and function but can be manufactured at scale and optimized beyond what evolution achieved. Their flagship product, XT-ViVo®, uses these biomimetic molecules to prevent ice recrystallization while maintaining tissue functionality across significant temperature variations below 0°C.
The results have been dramatic. In 2022, the FDA granted XT-ViVo® Breakthrough Device status for kidney preservation up to 120 hours—five times longer than current standards. The company recently completed the world's first subzero transatlantic organ transports, shipping pig kidneys from the United States to Vienna in portable TimeSeal® devices that require no external power or oxygen. After 48-72 hours of subzero preservation, the kidneys were successfully transplanted and maintained normal function for over 200 days.
"The technology is stream-lined, modifiable, and easy to use," explains Dr. Gerald Brandacher, who led the transplant team. "The transporter doesn't require external power, oxygen, or blood for the tissue or organ to survive." This portability could revolutionize transplant logistics, enabling global organ sharing and potentially the creation of organ banks where tissues are stored and matched to recipients on demand rather than in desperate real-time scrambles.
Research also revealed that insect AFPs outperform fish AFPs for certain applications. Using specialized microscopes with millidegree temperature control, Professor Ido Braslavsky demonstrated that "proteins in insects are much more efficient in inhibiting ice growth than proteins in fish, but fish proteins bind faster to ice." This suggests future cryopreservation cocktails might blend different AFP types, using fast-binding fish proteins for initial protection and highly efficient insect proteins for long-term storage.
Agriculture's Climate Adaptation
As climate change brings more frequent late-spring frosts and unpredictable temperature swings, crop losses from freeze damage are mounting. A single frost event can devastate entire harvests, costing billions globally. AFPs offer a genetic solution.
Researchers have successfully transferred AFP genes from Arctic flounder into strawberries, potatoes, and tobacco, creating transgenic plants that produce fish antifreeze proteins in their tissues. These proteins inhibit ice crystal formation in plant cells, extending cold tolerance by several degrees. Field trials of AFP-expressing strawberries showed survival at temperatures several degrees below lethal thresholds for unmodified plants. Frost-resistant soybeans withstood brief exposures to -4°C without significant yield losses.
Beyond direct gene transfer, scientists are also manipulating plants' native cold-response pathways. Overexpressing CBF (C-repeat binding factor) genes—master regulators of cold adaptation—in Arabidopsis produced plants surviving temperatures several degrees lower than wild-type. The advantage of this approach is that it activates entire networks of cold-protective genes rather than adding a single foreign protein, potentially conferring broader stress resistance.
But challenges remain. Constitutive AFP expression can trigger unintended consequences: delayed flowering, reduced growth rates, or metabolic costs that diminish yields. The key is conditional expression—activating AFP production only when temperatures drop, mimicking fish that regulate AFP synthesis seasonally. Combining AFPs with optimized membrane lipid compositions, stress-responsive gene networks, and traditional breeding for cold-hardy varieties may yield the most robust frost resistance without yield penalties.
The implications extend beyond preventing frost damage. As polar regions warm and temperate zones shift poleward, AFP-equipped crops could enable agriculture in currently marginal lands, buffering food security against climate disruption.
Food Science and Beyond
The food industry has quietly begun deploying AFPs to improve frozen product quality. Ice crystal growth during freeze-thaw cycles degrades texture in ice cream, frozen fruits, vegetables, and dough. AFPs prevent recrystallization, maintaining smooth textures and reducing drip loss during thawing.
Recent research with Antarctic yeast AFPs demonstrated practical benefits. When researchers added recombinant yeast AFP (GaAFP) to frozen carrots, kohlrabi, and blueberries, drip loss after 40 days of storage dropped from 28% (controls) to just 12-13%—matching the performance of glycerol, the industry-standard cryoprotectant. Ice crystals in AFP-treated samples remained under 5 micrometers even after 60 minutes at -8°C, while control crystals ballooned beyond 30 micrometers. The AFPs also protected baker's yeast cells during freezing, suggesting applications in preserving industrial microbial cultures.
The advantage of AFPs over chemical cryoprotectants is that they're proteins—biodegradable, non-toxic, and derived from natural sources. As consumers demand cleaner labels, AFPs offer a way to improve frozen food quality without synthetic additives. Yeast-derived AFPs can be produced in mesophilic hosts like Pichia pastoris at 28°C and purified using simple tangential flow filtration, making scalable commercial production feasible.
Beyond food, AFPs are inspiring ice-phobic coatings for aircraft wings and wind turbines, anti-icing fluids for infrastructure, and even materials for long-duration space travel where cryopreservation would be essential.
The potential applications of antifreeze proteins seem boundless, but their future is shadowed by the same forces that made them necessary: climate change.
Arctic sea ice extent has declined an average of 12.8% per decade between 1981 and 2010, with summer ice losses even more dramatic. As the Arctic warms, the very fish whose proteins we're harvesting for medical and agricultural breakthroughs face existential threats. The variegated snailfish discovered in Greenland's iceberg habitats—the one with record-breaking AFP expression—exemplifies this paradox. Its extraordinary adaptations allow survival in the most extreme niches, but those specializations may become liabilities as ice habitats shrink and temperate competitors move poleward.
"Warming oceanic temperatures could pose a threat to these highly specialized fishes, which may face increased competition from temperate species," warns John Sparks, curator at the American Museum of Natural History. Fish that evolved to dominate frozen seas may find themselves outcompeted in merely cold ones, their once-invaluable AFPs offering little advantage when sea temperatures rise above the narrow range where antifreeze matters.
There's also the unintended consequence problem. Antarctic fish carrying persistent internal ice crystals—protected from melting by the same AFPs that prevent freezing—may suffer physiological costs we don't yet understand. Could accumulated ice obstruct blood flow or trigger inflammatory responses? An 11-year temperature record from McMurdo Sound showed no warming events sufficient to melt this internal ice during fish lifespans, but as climate shifts, fish may experience novel thermal stresses that their billion-year-old adaptations can't handle.
Meanwhile, the race to commercialize AFP technology raises questions about intellectual property and access. If AFP-derived preservation methods become standard in transplant medicine, will they remain affordable and globally accessible, or become premium technologies available only in wealthy nations? If AFP-equipped crop varieties are patented by agricultural corporations, will smallholder farmers in developing countries—those most vulnerable to climate disruption—be able to afford them?
There's also the issue of unintended ecological consequences. Releasing AFP-expressing crops into agricultural ecosystems creates the possibility of gene flow to wild relatives, potentially creating invasive super-weeds with enhanced cold tolerance. Careful containment and monitoring will be essential.
The convergent evolution of antifreeze proteins across fish, insects, plants, and microbes illustrates a fundamental principle: similar problems have similar solutions, and the constraints of physics narrow the design space for biological innovation. But it also reveals evolution's creativity—the four independent origins of Type I AFPs from completely different genetic starting points show that there are multiple paths to the same destination.
This dual lesson—that nature's solutions are both universal and diverse—offers a roadmap for the challenges ahead. As we deploy AFP technology in medicine, agriculture, and industry, we should seek not to simply copy nature's solutions but to understand the principles underlying them, then engineer improvements suited to human needs.
Computational protein design is already enabling this. By modeling how threonine ladder geometry affects ice-binding efficiency across different β-solenoid scaffolds, researchers can now design synthetic AFPs that nucleate ice at predetermined temperatures or optimize specific properties like recrystallization inhibition. X-Therma's peptoids demonstrate that biomimetic molecules can outperform their natural inspirations.
The path forward requires integration across disciplines. Structural biologists decode AFP mechanisms. Computational scientists simulate protein-ice interactions. Geneticists transfer and optimize AFP expression. Clinicians test preservation protocols. Agronomists field-trial frost-resistant crops. Each advance builds on insights from organisms that solved these problems millions of years before humans existed.
Yet we must also recognize that we're drawing from a library that's actively burning. Every species driven extinct by climate change takes with it millions of years of evolutionary problem-solving—adaptations we haven't yet discovered, proteins we haven't yet sequenced, solutions to problems we haven't yet encountered. The variegated snailfish glowing faintly in Greenland's ice-choked waters isn't just a curiosity; it's a repository of molecular innovations that might one day save human lives or feed populations through climate catastrophes.
Standing at the intersection of evolutionary deep time and technological acceleration, we face a choice that will define this century: Will we learn from nature's frozen survivors quickly enough to preserve both them and ourselves?
The proteins that keep fish alive at -2°C are already extending the viability of human organs from hours to days, potentially saving thousands of lives annually. They're protecting crops from increasingly erratic frosts, buffering food security against climate chaos. They're improving frozen foods, inspiring ice-phobic materials, and expanding the boundaries of cryobiology.
But these applications only matter if we preserve the ecosystems and climate stability that allowed such adaptations to evolve. A warming Arctic doesn't just threaten polar bears and sea ice—it threatens the living libraries of evolutionary solutions we've barely begun to read.
The fish swimming beneath Arctic ice don't know they're performing molecular miracles. They don't know their blood proteins represent hundreds of millions of years of evolutionary trial and error, or that human researchers are studying them to revolutionize medicine and agriculture. They simply survive, as their ancestors have survived through ice ages and interglacials, carrying in their cells a molecular heritage older than our species.
What we do with that heritage—whether we cherish it as a gift from deep time or squander it through neglect and short-term thinking—will be our generation's defining legacy. The antifreeze proteins aren't just keeping fish alive in frozen seas. They're offering us a choice about what kind of future we want to build: one where we learn from nature's wisdom and work with evolutionary time, or one where we destroy our greatest teachers even as we're discovering what they have to teach.
The ice is melting. The clock is ticking. And the fish that defied physics to survive nature's frozen frontier are now showing us how we might survive our own.

Recent breakthroughs in fusion technology—including 351,000-gauss magnetic fields, AI-driven plasma diagnostics, and net energy gain at the National Ignition Facility—are transforming fusion propulsion from science fiction to engineering frontier. Scientists now have a realistic pathway to accelerate spacecraft to 10% of light speed, enabling a 43-year journey to Alpha Centauri. While challenges remain in miniaturization, neutron management, and sustained operation, the physics barriers have ...

Epigenetic clocks measure DNA methylation patterns to calculate biological age, which predicts disease risk up to 30 years before symptoms appear. Landmark studies show that accelerated epigenetic aging forecasts cardiovascular disease, diabetes, and neurodegeneration with remarkable accuracy. Lifestyle interventions—Mediterranean diet, structured exercise, quality sleep, stress management—can measurably reverse biological aging, reducing epigenetic age by 1-2 years within months. Commercial ...

Data centers consumed 415 terawatt-hours of electricity in 2024 and will nearly double that by 2030, driven by AI's insatiable energy appetite. Despite tech giants' renewable pledges, actual emissions are up to 662% higher than reported due to accounting loopholes. A digital pollution tax—similar to Europe's carbon border tariff—could finally force the industry to invest in efficiency technologies like liquid cooling, waste heat recovery, and time-matched renewable power, transforming volunta...

Humans are hardwired to see invisible agents—gods, ghosts, conspiracies—thanks to the Hyperactive Agency Detection Device (HADD), an evolutionary survival mechanism that favored false alarms over fatal misses. This cognitive bias, rooted in brain regions like the temporoparietal junction and medial prefrontal cortex, generates religious beliefs, animistic worldviews, and conspiracy theories across all cultures. Understanding HADD doesn't eliminate belief, but it helps us recognize when our pa...

The bombardier beetle has perfected a chemical defense system that human engineers are still trying to replicate: a two-chamber micro-combustion engine that mixes hydroquinone and hydrogen peroxide to create explosive 100°C sprays at up to 500 pulses per second, aimed with 270-degree precision. This tiny insect's biochemical marvel is inspiring revolutionary technologies in aerospace propulsion, pharmaceutical delivery, and fire suppression. By 2030, beetle-inspired systems could position sat...

The U.S. faces a catastrophic care worker shortage driven by poverty-level wages, overwhelming burnout, and systemic undervaluation. With 99% of nursing homes hiring and 9.7 million openings projected by 2034, the crisis threatens patient safety, family stability, and economic productivity. Evidence-based solutions—wage reforms, streamlined training, technology integration, and policy enforcement—exist and work, but require sustained political will and cultural recognition that caregiving is ...

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.