Digital Pollution Tax: Can It Save Data Centers?

TL;DR: Scientists have engineered bacteria that can digest plastic waste by breaking down PET polymers into reusable monomers, enabling infinite recycling cycles instead of the typical three to five. Pilot plants are already processing hundreds of tons of plastic annually, with costs now lower than producing virgin plastic. While the technology promises to transform waste management, landfills, and ocean cleanup, deployment faces ecological risks, regulatory hurdles, and the challenge of scaling enzyme production. Within a decade, bacterial recycling could become commonplace—if society navigates the trade-offs wisely.

In 2016, scientists at a Japanese recycling facility noticed something extraordinary: polyethylene terephthalate bottles—the kind that take centuries to decompose—were disappearing. The culprit wasn't a chemical process or an incinerator. It was a bacterium, Ideonella sakaiensis, eating the plastic and using it as fuel. Within the next decade, this discovery could transform every landfill, wastewater plant, and ocean cleanup operation on Earth. The question isn't whether bacteria can digest plastic—we know they can. The question is whether we'll deploy them before we drown in 400 million tons of new plastic waste each year.
When Professor Kohei Oda's team isolated Ideonella sakaiensis from sediment at a bottle recycling plant, they discovered that roughly 75% of the PET plastic in their samples had degraded to CO₂. This wasn't slow decomposition over decades—it was active digestion. The bacterium secretes two enzymes, PETase and MHETase, that work in tandem like molecular scissors. PETase first cleaves the long polymer chains of PET into smaller fragments called MHET (mono(2-hydroxyethyl) terephthalic acid), then MHETase breaks MHET down further into terephthalic acid and ethylene glycol—the same monomers used to manufacture virgin plastic.
What makes this discovery revolutionary isn't just that bacteria can eat plastic. It's that they can convert it into metabolically useful compounds, channeling the carbon into their own cells or releasing it as CO₂. This two-enzyme cascade represents a naturally evolved pathway for plastic upcycling—a circular economy happening at the microbial level. Under ideal laboratory conditions, wild-type I. sakaiensis can break down a 0.2 mm thin film of low-crystallinity PET in approximately six weeks. High-crystallinity PET takes longer—about 180 weeks—but the principle holds: what humanity synthesized over the past 70 years, evolution has already begun to dismantle.
The discovery arrived at a critical moment. By 2016, global plastic production had reached 335 million metric tons annually, with only 9% being recycled and most of the rest ending up in landfills or the ocean. Traditional recycling methods—mechanical shredding and melting—degrade plastic quality with each cycle, limiting reuse to three to five iterations before the material becomes unusable. Enzymatic digestion, by contrast, breaks plastic down to its molecular building blocks, enabling theoretically infinite recycling. The implications were immediate: if bacteria could be engineered to work faster and at scale, they could provide a biological solution to one of the most intractable pollution crises of the Anthropocene.
Humanity has faced material crises before, and biology has often provided the answer. In the early 20th century, industrial nitrogen fixation—mimicking what soil bacteria do naturally—enabled the Green Revolution and fed billions. In 1978, scientists engineered Escherichia coli to produce synthetic human insulin, solving allergic reactions to animal-derived insulin and launching the biotechnology industry. Each breakthrough followed a pattern: identify a natural biological process, isolate the molecular machinery, and scale it for industrial use.
Plastic-eating bacteria follow this same trajectory. The enzymes responsible—PETase and MHETase—are hydrolases, a class of enzymes that use water to break chemical bonds. Nature has been using hydrolases to decompose organic matter for billions of years; what's new is their application to synthetic polymers. The fact that I. sakaiensis evolved PETase so recently—PET was only invented in 1941—suggests that microbial metabolism is already adapting to the Plasticene, the geological layer of plastic waste accumulating in sediments worldwide. This rapid evolution hints at a deeper truth: plastics may not be as durable as we assumed. The materials we designed to last forever are, in evolutionary terms, just another carbon source waiting to be exploited.
History also teaches caution. The introduction of synthetic pesticides in the 1940s promised to eliminate agricultural pests, but within decades, resistance had emerged and ecosystems were damaged. Similarly, deploying engineered bacteria into the environment without understanding their ecological impact could create unintended consequences—horizontal gene transfer to wild populations, disruption of microbial communities, or the evolution of plastic-degrading pathogens. The lesson from DDT and other agrochemicals is clear: biological tools are powerful, but their systemic effects require rigorous assessment. The same applies to plastic-eating bacteria.
Yet the potential is undeniable. Just as the printing press democratized knowledge and the steam engine mechanized labor, engineered bacteria could decentralize waste management. Imagine portable bioreactors deployed in remote areas, wastewater plants that digest microplastics before discharge, or landfills seeded with bacteria that convert plastic into compost. The infrastructure already exists; the challenge is integrating biological processes into it. History suggests that when biology and engineering converge, the results reshape civilization. The question is whether we'll learn from past mistakes or repeat them.
At the molecular level, PET is a polymer—a long chain of repeating units held together by ester bonds. These bonds are stable, which is why PET bottles can sit in landfills for centuries without breaking down. PETase works by positioning itself on the plastic surface and catalyzing hydrolysis, using water molecules to cleave the ester bonds. The enzyme's active site is physically tailored to bind to PET, with a structure that allows it to operate at ambient temperatures around 30°C—ideal for biological reactors and far more energy-efficient than thermal or chemical recycling.
Once PETase breaks PET into MHET and smaller oligomers like BHET (bis(2-hydroxyethyl) terephthalic acid), MHETase completes the job. This second enzyme hydrolyzes MHET into terephthalic acid (TPA) and ethylene glycol (EG), both of which are water-soluble and can enter the bacterium's central metabolism. Inside the cell, TPA is transported by a protein called PcaK and converted into protocatechuic acid, which feeds into the tricarboxylic acid (TCA) cycle—the same pathway that powers all aerobic life. Essentially, the bacterium treats plastic as food, extracting energy and carbon just as it would from sugars or fatty acids.
Engineers have since turbocharged this process. In 2018, researchers at the University of Portsmouth and the U.S. Department of Energy's National Renewable Energy Laboratory (NREL) created an enhanced PETase variant that degrades plastic 20% faster than the natural enzyme. By 2020, they developed a hybrid enzyme combining PETase and MHETase into a single polypeptide, boosting degradation rates sixfold. The latest iteration, FAST-PETase (functional, active, stable, and tolerant PETase), was designed using machine learning. Researchers at the University of Texas at Austin fed structural data from 51 post-consumer plastic containers into an AI model, generating novel mutations that increase enzyme stability and activity. FAST-PETase can depolymerize PET in as little as 24 hours under optimal conditions and remains active at temperatures below 50°C, making it suitable for field deployment in landfills and waste facilities.
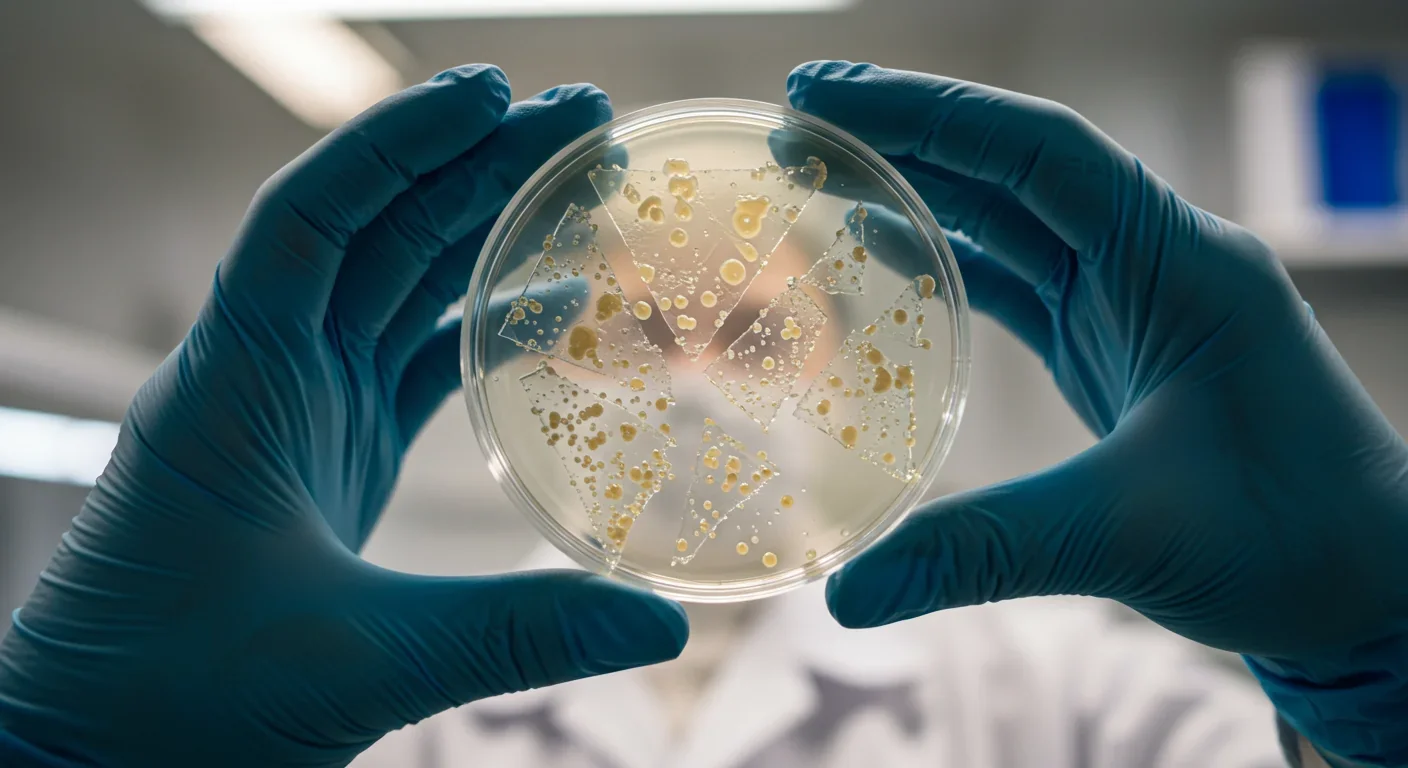
But engineering the enzyme is only half the challenge. To deploy bacteria at scale, scientists must optimize the entire system—enzyme production, secretion, stability, and recovery of monomers. One breakthrough involves surface display: anchoring PETase to the outside of bacterial cells using protein scaffolds like curli fibers. This approach, called BIND-PETase, keeps the enzyme close to the plastic surface, enhances substrate access, and allows the bacteria to be reused. In one experiment, BIND-PETase remained active for over a month at 4°C, suggesting it could function in cold environments like unheated warehouses or northern-latitude landfills. Another innovation pairs surface-displayed PETase with intracellular MHETase, creating a synergistic system that reduces product inhibition—a major bottleneck where accumulated MHET slows down the reaction. This combination increased BHET degradation 10- to 20-fold compared to free enzymes.
If engineered bacteria become the backbone of plastic waste management, the ripple effects will touch nearly every industry. The global recycling sector—currently valued at over $50 billion and growing—will shift from mechanical sorting and melting to biological processing. Wastewater treatment plants, which already host trillions of bacteria, could become plastic remediation hubs. Cities might install enzymatic reactors alongside sewage digesters, capturing microplastics before they reach rivers and oceans. Landfills, often seen as environmental liabilities, could transform into resource recovery sites where plastic is mined, digested, and repolymerized into virgin-quality material.
The apparel and packaging industries will feel the change acutely. Fast fashion and single-use plastics thrive on cheap virgin polyester, but enzymatic recycling could flip the economics. French biotech company Carbios has demonstrated that its engineered cutinase enzyme can process any PET product—bottles, food packaging, textiles—and recover 97% of the constituent monomers. Unlike mechanical recycling, which downgrades material quality, Carbios' process yields monomers indistinguishable from petroleum-derived feedstock, enabling 30 to 50 recycling cycles versus the current three to five. Major brands including Nestlé Waters, PepsiCo, L'Oréal, Patagonia, Puma, and Salomon have already partnered with Carbios, signaling market readiness. In 2021, Carbios unveiled a demonstration plant in France processing hundreds of tons of PET annually; a 50,000-ton commercial facility capable of recycling 2 billion bottles or 300 million T-shirts is planned for 2028.
Job markets will evolve in tandem. Demand will grow for biochemists, genetic engineers, and microbial ecologists—roles that barely existed a generation ago. At the same time, traditional waste-sorting jobs may decline as biological systems automate degradation. The transition will mirror the shift from coal mining to renewable energy: painful for some communities, transformative for others. Governments will need retraining programs, just as they did when agriculture mechanized or factories automated.
Culturally, widespread bacterial recycling could reshape how society views waste. Today, throwing away a plastic bottle is an act of finality—out of sight, out of mind. But if that bottle can be enzymatically digested, repolymerized, and returned to the supply chain within weeks, waste becomes a temporary state rather than a permanent one. This shift in perspective could accelerate circular economy thinking, where products are designed not for disposal but for infinite reuse. It might also reduce the stigma around "recycled" goods, especially if enzymatically recycled plastic is chemically identical to virgin material. Fashion designer Stella McCartney has already showcased a jacket made from Protein Evolution's biologically recycled polyester at COP 28, branding it "the first garment ever made using biological recycling." Such high-profile endorsements signal that biologically recycled materials could become aspirational, not inferior.
The benefits of bacterial plastic digestion extend far beyond waste management. Consider microplastics—fragments smaller than 5 millimeters that pervade oceans, soil, drinking water, and even human bloodstreams. Mechanical recycling can't capture them, and incineration releases toxic fumes. Engineered bacteria, however, can target microplastics in situ. Researchers at the University of Waterloo have developed a system using "bacterial sex"—horizontal gene transfer via conjugation—to spread plastic-degrading genes among wastewater bacteria. This approach leverages bacteria that naturally dominate wastewater treatment plants, turning them into microplastic scavengers without requiring genetic modification of wild populations. Because wastewater facilities are designed to neutralize bacteria before discharge, the risk of environmental escape is minimized.
Another promise is upcycling: converting plastic waste into valuable chemicals. Scientists at the University of Edinburgh genetically engineered E. coli to digest terephthalic acid (a PET breakdown product) and synthesize paracetamol, a common painkiller. Other teams are engineering bacteria to produce vanillin (vanilla flavoring), muconic acid (a precursor for nylon and other plastics), or medium-chain-length polyhydroxyalkanoates (biodegradable bioplastics). This transforms waste from a liability into a feedstock for high-value goods—an economic inversion that could make plastic recycling not just sustainable but profitable.
Energy savings are equally compelling. Enzymatic recycling operates at low temperatures (often below 50°C) and atmospheric pressure, slashing energy consumption compared to traditional methods. A recent breakthrough by NREL and the University of Portsmouth replaced sodium hydroxide with ammonium hydroxide in the enzymatic process, cutting chemical use by 99% and energy consumption by 65%. The ammonium regenerates itself through thermolysis of a byproduct, creating a closed-loop system. The result: recycled PET costs $1.51 per kilogram—cheaper than virgin plastic at $1.87 per kilogram. When recycling becomes cheaper than producing new material, market forces will drive adoption without requiring subsidies or mandates.
Finally, bacterial degradation could address plastics beyond PET. While polyethylene (PE) and polypropylene (PP)—polyolefins that lack ester bonds—are harder to digest, researchers are making progress. Marine bacteria like Rhodococcus ruber form biofilms on polyethylene surfaces and secrete enzymes such as alkane hydroxylase and laccase to initiate degradation. Mixed bacterial-fungal consortia have achieved 55.6% degradation of low-density polyethylene (LDPE) in 90 days, outperforming single-species cultures. A 2021 study led by Jan Zrimec identified 30,000 non-redundant plastic-degrading enzyme homologs from marine microbiomes, suggesting a vast untapped enzymatic repertoire. If even a fraction of these can be engineered for industrial use, the range of biodegradable plastics will expand dramatically.
For all its promise, deploying engineered bacteria at scale raises profound concerns. The most immediate is ecological risk. Releasing genetically modified organisms (GMOs) into the environment—even in controlled settings like wastewater plants—could lead to unintended gene transfer. Bacteria engage in horizontal gene transfer, swapping genetic material through conjugation, transformation, or transduction. If a plastic-degrading gene jumps to a pathogenic species, the result could be unpredictable. One study found that Pseudomonas aeruginosa, a deadly hospital superbug, naturally produces an enzyme called Pap1 that degrades polycaprolactone (PCL)—a biodegradable plastic used in medical devices like stents, sutures, and wound dressings. In lab tests, Pap1 degraded 78% of PCL in just seven days. More alarming, broken-down plastic fragments help the bacteria form tougher biofilms—protective slime coatings that increase antibiotic resistance. If plastic-eating genes spread to clinical isolates of P. aeruginosa, hospitals could face devices that degrade while still implanted, or infections that are harder to treat.
Containment is another challenge. While wastewater plants offer built-in neutralization (bacteria are typically killed before discharge), landfills and open environments do not. If engineered bacteria escape and colonize natural ecosystems, they could disrupt microbial communities that play critical roles in nutrient cycling, soil health, and plant growth. The potential for "genetic pollution"—the spread of engineered traits to wild populations—has led regulatory agencies to demand rigorous risk assessments. In the United States, the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA), and the U.S. Department of Agriculture (USDA) share oversight of GMOs, with each agency applying different standards depending on the organism's use. Approving an engineered microbe for landfill deployment would require coordination across all three, plus compliance with the Coordinated Framework for Regulation of Biotechnology—a process that can take years.
Economic viability remains uncertain. While enzymatic recycling has achieved cost parity with virgin plastic in some pilot studies, scaling to millions of tons per year is another matter. Enzyme production is expensive: current techno-economic analyses estimate that enzyme cost accounts for roughly 4% of total recycling cost, but these figures assume production levels comparable to cellulases (enzymes used in biofuel production). Achieving such scale requires massive bioreactor infrastructure. Solid-state fermentation using agricultural byproducts could reduce costs by up to tenfold, but this technology is still in early development. Protein Evolution, a startup using AI to design plastic-eating enzymes, has raised $25 million and plans a pilot plant producing 300 tons of recycled polyester per year by 2025, followed by a 50,000-ton commercial facility in 2028. Whether these timelines hold—and whether the economics work at scale—will determine the technology's viability.

Then there's the problem of substrate specificity. Most engineered PETases only work on PET, which accounts for roughly 8% of global plastic production. Polyethylene and polypropylene—used in bags, containers, and packaging—together represent over 50% of plastic waste, and they're far more resistant to enzymatic attack due to their carbon-carbon backbone and lack of ester bonds. Enzymes like alkane hydroxylase can initiate polyolefin degradation, but the process is slow and inefficient. Until researchers develop robust enzymes for PE and PP, bacterial recycling will remain a partial solution.
Finally, there's the risk of public backlash. GMOs face deep skepticism, especially in Europe, where regulatory frameworks are among the world's strictest. Deploying engineered bacteria in waste facilities—even with containment measures—could trigger opposition from environmental groups and the public. The lesson from genetically modified crops is that scientific consensus does not guarantee social acceptance. Building trust will require transparency, rigorous safety testing, and proactive engagement with communities. If the technology is perceived as rushed or imposed without consultation, resistance could delay or derail deployment.
The race to deploy plastic-eating bacteria is global, but approaches vary sharply by region. In Asia, where plastic waste generation is highest, governments are investing heavily in biotechnology solutions. Japan, home to the original Ideonella sakaiensis discovery, leads in enzyme research and has partnered with universities and companies to pilot enzymatic recycling. China, the world's largest plastic producer and waste generator, has launched initiatives to integrate microbial degradation into its waste management infrastructure, particularly in wastewater treatment. Singapore, constrained by limited land for landfills, is exploring enzymatic recycling as part of its circular economy strategy.
In Europe, the emphasis is on regulatory rigor and sustainability. The European Union requires that any material labeled "biodegradable" must convert more than 90% into CO₂, water, and minerals within six months under standardized conditions—a benchmark that sets a high bar for bacterial degradation. France, where Carbios is based, has positioned itself as a leader in enzymatic recycling, with government support for pilot plants and partnerships with major brands. The EU's strict GMO regulations, however, mean that deploying engineered bacteria in open environments will face extensive scrutiny and approval processes that could take years.
In North America, the approach blends innovation with market-driven pragmatism. The United States has a more permissive regulatory environment for GMOs compared to Europe, which has enabled rapid development of engineered bacteria. Protein Evolution, based in the U.S., secured venture funding and is racing toward commercial-scale deployment by 2028. Canada's University of Waterloo team has pioneered the use of bacterial conjugation to spread plastic-degrading genes in wastewater bacteria, leveraging existing infrastructure. The North American model favors speed and scalability, with less emphasis on precautionary regulation.
Developing nations face a different calculus. Countries in Africa, Latin America, and Southeast Asia often lack the infrastructure for mechanical recycling, let alone advanced enzymatic systems. Yet they also bear a disproportionate burden of plastic pollution, with waste imported from wealthier nations and inadequate disposal systems. For these regions, portable, low-cost bioreactors could be transformative. Researchers at Penn State are collaborating with Duke University to develop a solar-powered bioreactor for field deployment in areas with limited electricity and waste infrastructure. If successful, such systems could leapfrog traditional recycling, much as mobile phones bypassed landlines in many developing countries.
International cooperation—or competition—will shape the technology's trajectory. The discovery of plastic-eating enzymes is a global commons, with research published openly, but commercialization is competitive. Companies like Carbios and Protein Evolution are filing patents, and first-mover advantage could determine market dominance. Whether bacterial recycling becomes a tool for global equity or another vector for technological inequality depends on how intellectual property is managed and whether low-income nations gain access to the technology.
As bacterial plastic digestion moves from lab to landfill, individuals and institutions must adapt. For students and career-changers, the bioeconomy offers growing opportunities. Demand is rising for synthetic biologists, protein engineers, and bioinformaticians who can design and optimize enzymes using tools like CRISPR and AI-driven protein folding models such as AlphaFold. Environmental scientists and chemical engineers will be needed to integrate biological systems into industrial processes. Policy experts and regulatory specialists will navigate the complex approval landscape for GMOs. Retraining programs, university curricula, and online courses in biotechnology are already expanding to meet this demand.
For businesses, the shift toward enzymatic recycling presents both risk and opportunity. Companies in the plastics value chain—manufacturers, packaging firms, apparel brands—must evaluate whether to partner with biotech startups, invest in in-house R&D, or wait for the technology to mature. Early adopters like PepsiCo and L'Oréal are positioning themselves as sustainability leaders, but laggards risk obsolescence if regulations or consumer preferences shift rapidly. Firms that produce hard-to-recycle plastics—multilayer packaging, mixed-material composites—face particular pressure. Carbios has noted that food packaging with three to twelve layers of different plastics represents a major bottleneck, and standardization to simpler, enzyme-compatible materials may become necessary.
Governments must balance innovation with oversight. Regulatory frameworks designed for 20th-century technologies are ill-suited to genetically engineered microbes deployed at environmental scale. The EPA, FDA, and USDA in the U.S. have released a web-based tool to help developers navigate the approval process for GMOs, and the USDA has issued a request for information to explore less burdensome pathways for commercializing genetically modified microbes. Streamlining regulation without compromising safety is a delicate task, requiring input from scientists, industry, environmental groups, and the public.
For citizens, understanding the trade-offs is essential. Bacterial recycling is not a silver bullet; it won't eliminate the need to reduce plastic consumption or improve product design. But it could significantly reduce the volume of plastic entering landfills and oceans, buy time while society transitions to alternative materials, and create economic value from waste. Public support—or opposition—will shape whether engineered bacteria are deployed widely or remain a niche technology. Engaging with the science, asking critical questions, and participating in public comment periods on regulatory proposals are all forms of civic action.
Skills to develop include data literacy (to evaluate claims about enzyme efficiency and environmental impact), systems thinking (to understand how biological interventions interact with infrastructure and ecosystems), and ethical reasoning (to weigh the benefits of pollution reduction against the risks of releasing GMOs). The future of plastic waste management will be decided not by scientists alone, but by society's collective choices.
The story of plastic-eating bacteria is, at its core, a story about human ingenuity confronting human consequences. We invented plastics to solve problems—durable, lightweight materials that revolutionized packaging, medicine, and manufacturing. Now, 400 million tons of plastic later, we're engineering life itself to clean up the mess. The technology works: enzymes can digest plastic, bacteria can be optimized to do it faster, and pilot plants are proving the concept at scale. Within a decade, enzymatic recycling could be commonplace, turning landfills into resource mines and wastewater plants into purification hubs.
Yet technology alone won't save us. Deploying engineered bacteria requires navigating ecological risks, regulatory mazes, economic uncertainties, and public skepticism. It demands coordination across governments, industries, and communities. It forces us to ask: What kind of future do we want—one where waste disappears into bacterial digestion, or one where we design materials that never become waste in the first place?
The answer is likely both. Bacterial recycling can address the legacy plastic already in the environment while we transition to biodegradable alternatives and circular design. But the transition must be intentional. Left unmanaged, engineered bacteria could create new problems—escaped genes, disrupted ecosystems, unequal access to the technology. Managed wisely, they could help bend the curve on plastic pollution, preserve ecosystems, and demonstrate that biology and engineering, working together, can reverse even the deepest scars we've left on the planet.
The choice is ours. The bacteria are ready. The question is whether we are.

Recent breakthroughs in fusion technology—including 351,000-gauss magnetic fields, AI-driven plasma diagnostics, and net energy gain at the National Ignition Facility—are transforming fusion propulsion from science fiction to engineering frontier. Scientists now have a realistic pathway to accelerate spacecraft to 10% of light speed, enabling a 43-year journey to Alpha Centauri. While challenges remain in miniaturization, neutron management, and sustained operation, the physics barriers have ...

Epigenetic clocks measure DNA methylation patterns to calculate biological age, which predicts disease risk up to 30 years before symptoms appear. Landmark studies show that accelerated epigenetic aging forecasts cardiovascular disease, diabetes, and neurodegeneration with remarkable accuracy. Lifestyle interventions—Mediterranean diet, structured exercise, quality sleep, stress management—can measurably reverse biological aging, reducing epigenetic age by 1-2 years within months. Commercial ...

Data centers consumed 415 terawatt-hours of electricity in 2024 and will nearly double that by 2030, driven by AI's insatiable energy appetite. Despite tech giants' renewable pledges, actual emissions are up to 662% higher than reported due to accounting loopholes. A digital pollution tax—similar to Europe's carbon border tariff—could finally force the industry to invest in efficiency technologies like liquid cooling, waste heat recovery, and time-matched renewable power, transforming volunta...

Humans are hardwired to see invisible agents—gods, ghosts, conspiracies—thanks to the Hyperactive Agency Detection Device (HADD), an evolutionary survival mechanism that favored false alarms over fatal misses. This cognitive bias, rooted in brain regions like the temporoparietal junction and medial prefrontal cortex, generates religious beliefs, animistic worldviews, and conspiracy theories across all cultures. Understanding HADD doesn't eliminate belief, but it helps us recognize when our pa...

The bombardier beetle has perfected a chemical defense system that human engineers are still trying to replicate: a two-chamber micro-combustion engine that mixes hydroquinone and hydrogen peroxide to create explosive 100°C sprays at up to 500 pulses per second, aimed with 270-degree precision. This tiny insect's biochemical marvel is inspiring revolutionary technologies in aerospace propulsion, pharmaceutical delivery, and fire suppression. By 2030, beetle-inspired systems could position sat...

The U.S. faces a catastrophic care worker shortage driven by poverty-level wages, overwhelming burnout, and systemic undervaluation. With 99% of nursing homes hiring and 9.7 million openings projected by 2034, the crisis threatens patient safety, family stability, and economic productivity. Evidence-based solutions—wage reforms, streamlined training, technology integration, and policy enforcement—exist and work, but require sustained political will and cultural recognition that caregiving is ...

Every major AI model was trained on copyrighted text scraped without permission, triggering billion-dollar lawsuits and forcing a reckoning between innovation and creator rights. The future depends on finding balance between transformative AI development and fair compensation for the people whose work fuels it.