Why Crows Bring Gifts: The Science of Avian Intelligence

TL;DR: Scientists have discovered that organisms can inherit traits through protein structures called prions, not just DNA. This protein-based inheritance explains how memories, behaviors, and disease risks pass between generations through molecular shapes that copy themselves, fundamentally rewriting our understanding of heredity and evolution.

For over a century, biology's central dogma has been clear: DNA holds all the instructions for life, passing genetic information from one generation to the next through a linear sequence of nucleotides. But what if inheritance isn't just about genes? What if organisms can pass down memories, traits, and behaviors through something entirely different—proteins that fold into shapes, copy themselves, and transmit information across generations without touching DNA at all?
This isn't science fiction. It's prion-like protein inheritance, and it's rewriting the rules of heredity in ways that challenge everything we thought we knew about evolution, disease, and what it means to inherit.
The story begins with yeast. In 1965, geneticist Brian Cox observed something strange in his yeast cultures: a trait called [PSI+] that behaved like it was inherited, but couldn't be explained by any DNA mutation. It took three decades for scientists to figure out what was happening. Reed Wickner finally cracked the puzzle in 1994, proposing that [PSI+] wasn't caused by genes at all—it was caused by proteins that could change shape and force other proteins to do the same.
These weren't ordinary proteins. They were prions, notorious for causing devastating brain diseases like mad cow and Creutzfeldt-Jakob disease. But in yeast, they weren't killers. They were memory keepers, carriers of information that existed entirely outside the genetic code. The protein Sup35p could fold into a special shape—an amyloid—that was sticky, self-perpetuating, and heritable. When it touched a normal Sup35p molecule, it forced that protein to adopt the same misfolded shape, creating a chain reaction that spread through cells and into offspring.
What Cox had witnessed wasn't a genetic quirk. It was a fundamentally different mechanism of inheritance, one that operates through protein structure rather than DNA sequence.
Understanding prion-like inheritance requires rethinking what it means to store and transmit biological information. DNA works like a digital code: four letters (A, T, G, C) arranged in specific sequences that cells read and translate into proteins. But proteins themselves have a second layer of information encoded not in their sequence, but in their three-dimensional shape.
Most proteins fold into stable structures determined by their amino acid sequence. But some proteins—particularly those rich in glutamine and asparagine residues—can exist in multiple stable shapes. These are prion-forming proteins, and their ability to switch between conformations is what makes them so remarkable. One shape might be soluble and functional, while another forms rigid fibers called amyloids that stack together like bricks.
The magic happens when these amyloid forms touch normal proteins. The amyloid acts as a template, forcing the normal protein to refold into the same amyloid shape. This process—called templating—is self-sustaining. Each newly converted protein becomes a template for others, creating an exponential cascade. The amyloid structure grows, gets divided when cells divide, and passes into daughter cells. No DNA involved. Just protein copying protein.
In yeast, the Sup35 protein does exactly this. Its normal job is to help cells terminate protein synthesis properly. But when Sup35 switches to its prion form, it aggregates into amyloid fibers, sequestering the functional protein. This changes how cells read stop codons in genetic messages, creating new traits that can persist for hundreds of generations—all because of a protein's shape, not a gene's sequence.
Scientists have now identified dozens of prion-forming proteins in yeast, each capable of creating distinct heritable states. Some provide stress resistance, others alter metabolism, and a few even help yeast adapt to new environments. These proteins don't just exist; they function as an alternative genetic system running parallel to DNA.
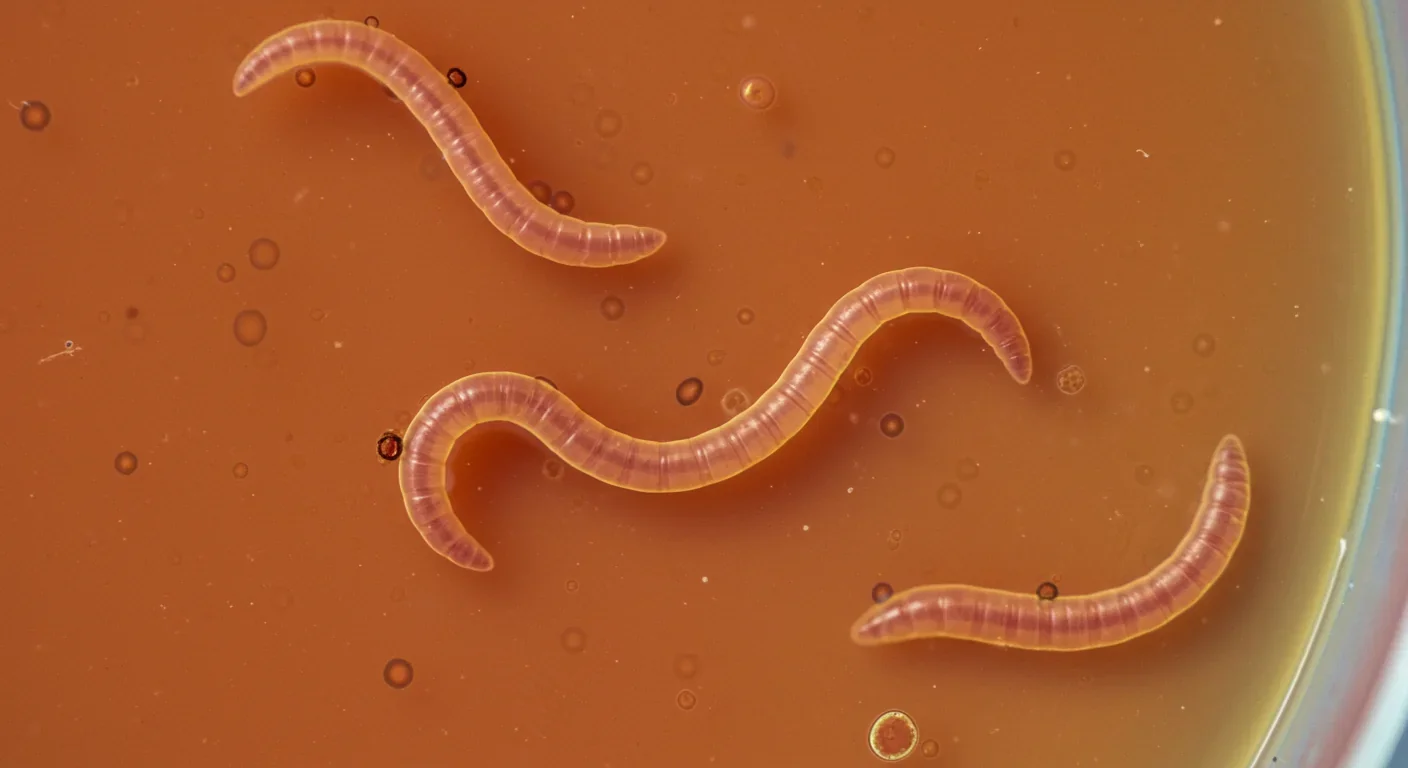
The discovery that yeast could inherit traits through proteins was striking, but yeast are simple single-celled organisms. Could protein-based inheritance work in complex multicellular animals? That question was answered in one of the most astonishing experiments in modern biology.
Researchers at the University of Toronto were studying cancer signaling in microscopic worms called C. elegans when they noticed something odd. The worms were producing more female offspring than normal, and the trait persisted generation after generation. The scientists initially suspected a genetic mutation, but sequencing the worms' DNA revealed nothing unusual. No gene had changed.
Digging deeper, they found the culprit: amyloid protein aggregates inside the worms' cells. These glowing green structures, which they named "herasomes," were disrupting a protein cycle that determines whether worms produce sperm or eggs. The amyloids were forcing the cycle to favor egg production, feminizing entire lineages. But the smoking gun came when researchers extracted these amyloids and injected them into normal worms. The recipient worms became feminized too, and they passed the trait to their offspring—all without any change to DNA or RNA.
"This is a new mechanism on top of genes that could explain part of our missing heritability," said Matthew Eroglu, one of the study's authors, pointing to the longstanding puzzle of why traits run in families even when no DNA differences can be found.
The implications were staggering. If worms could inherit traits through proteins, what about other animals? What about humans?
The answer might lie in memory itself. In the marine snail Aplysia, neuroscientist Eric Kandel discovered that a protein called CPEB—crucial for forming long-term memories—can adopt a prion-like state. When CPEB switches to this self-perpetuating form, it stabilizes connections between neurons, making memories last. Even more remarkable, research suggests these protein states might be transmissible. Inject extracts from trained snails into naive ones, and the naive snails can acquire behaviors they never learned, as if the memory itself had been transferred through protein.
This isn't just about worms or snails. Proteins with prion-like properties have been found in mammalian brains, including humans, where they help maintain synaptic strength—the physical basis of memory. The idea that memories could have a molecular form that persists beyond individual experience opens up profound questions about consciousness, learning, and what we inherit from our ancestors.
Not all prion-like inheritance is benign. The same molecular mechanism that allows yeast to adapt and neurons to remember can also cause some of the most terrifying diseases known to medicine.
Prion diseases—officially called transmissible spongiform encephalopathies—occur when normal brain proteins misfold into prion forms and spread through neural tissue. In humans, this includes Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru. In animals, it causes mad cow disease, chronic wasting disease in deer, and scrapie in sheep. All share a horrifying feature: they turn brains into sponge-like tissue, destroying neurons and causing dementia, movement disorders, and death. There's no cure, no treatment, and no way to stop the spread once it begins.
The difference between helpful yeast prions and deadly human prions lies in where they act and what they do. Yeast prions typically aggregate in the cytoplasm, affecting metabolic proteins in ways that might be reversible or even adaptive. Mammalian prion diseases involve proteins like PrP that misfold in the brain, accumulating in extracellular plaques that are toxic to neurons. The pathology is irreversible.
But the connection between prions and neurodegenerative disease goes deeper. Amyloid plaques—the hallmark of Alzheimer's disease—are structurally similar to prion aggregates. So are the tau tangles in Alzheimer's and the alpha-synuclein clumps in Parkinson's. These diseases aren't officially classified as prion diseases, but they share key features: misfolded proteins that aggregate, spread from cell to cell, and propagate their shape through templating.
Recent research has shown that Alzheimer's and Parkinson's pathology can spread through brain tissue in patterns consistent with prion-like transmission. Inject brain tissue containing misfolded tau or alpha-synuclein into healthy animals, and the pathology spreads. This doesn't mean these diseases are contagious in the traditional sense, but it does mean the underlying mechanism—protein-based information transfer—is the same as in classical prion diseases.
If neurodegenerative diseases involve prion-like processes, it fundamentally changes how we think about treatment. Instead of focusing solely on clearing plaques or preventing protein production, we might need to target the templating process itself: blocking the conversion of normal proteins to pathological forms, disrupting amyloid growth, or enhancing cellular machinery that clears aggregates.

The discovery of protein-based inheritance has profound implications for evolutionary biology. Since Darwin, evolution has been understood as the gradual accumulation of genetic mutations shaped by natural selection. Organisms with beneficial mutations survive and reproduce, passing advantageous genes to their offspring. This process takes many generations and requires permanent changes to DNA.
But prion-like inheritance offers an entirely different mode of adaptation—one that's faster, reversible, and responsive to the environment in ways DNA never could be.
Consider the worm feminization study. The amyloid-driven trait appeared suddenly, persisted for multiple generations, and then disappeared when environmental conditions changed. Researchers found that heat stress accelerated feminization, while cooler temperatures reversed it. This is phenotypic plasticity on steroids: organisms can rapidly adopt new traits, test them across generations, and abandon them if they're not useful—all without waiting for random mutations or risking permanent genetic changes.
In yeast, prion-based inheritance allows populations to hedge their bets. When conditions are stable, most cells maintain normal protein conformations. But when stress hits—starvation, temperature shock, toxic chemicals—some cells switch to prion states that alter metabolism, change nutrient preferences, or increase stress resistance. If the prion state helps, those cells survive and the trait spreads. If not, cells can revert to normal. It's a form of evolutionary experimentation that happens in real time, not over millennia.
This challenges the gene-centric view of evolution. Traits don't have to be encoded in DNA to be heritable or adaptive. Protein conformations can serve as a parallel inheritance system, transmitting information that responds to environmental signals and influences survival—all the hallmarks of evolutionary adaptation, just operating through a different molecular substrate.
The concept also intersects with epigenetics, the study of heritable changes that don't involve DNA sequence alterations. Histone modifications, DNA methylation, and other epigenetic marks can regulate which genes are turned on or off and can be passed to offspring. Recent research from UC Santa Cruz showed that specific histone modifications—proteins that package DNA—can be inherited across multiple generations in worms, affecting gene expression and development. The line between epigenetic inheritance and prion-like inheritance is blurring; both involve protein-based information transfer that operates outside the genetic code.
Some scientists now propose that non-genetic inheritance mechanisms might be crucial for rapid adaptation to climate change, pollution, and other environmental disruptions happening too fast for traditional evolution to address. If organisms can acquire and pass down adaptive protein states within a few generations, it could buy time for populations to survive while slower genetic adaptations catch up.
For decades, geneticists have wrestled with a puzzle called "missing heritability." Many traits and diseases clearly run in families—height, intelligence, diabetes, heart disease, schizophrenia—but when scientists search for the genes responsible, they come up short. Even when hundreds of genetic variants are identified, they only explain a fraction of the heritability observed in populations. The rest is missing.
Part of the answer might be environmental factors, gene-gene interactions, or rare variants that studies miss. But another part could be inheritance mechanisms that don't involve DNA at all.
Prion-like protein inheritance fits the profile. It's heritable, it influences traits, and it's invisible to standard genetic sequencing. If proteins can transmit information across generations, they could account for some of the familial clustering of diseases like Type 2 diabetes, obesity, and even aspects of behavior and cognition that seem to run in families but lack clear genetic explanations.
Consider Alzheimer's disease. Most cases aren't explained by known genetic mutations. Family history is a strong risk factor, yet the genes identified so far account for only a small fraction of that risk. If amyloid proteins can adopt prion-like states and be transmitted from parent to offspring—perhaps through the germline, or even behaviorally—it could explain why Alzheimer's clusters in families without obvious genetic causes.
The same logic applies to metabolic diseases. If parental diet, stress, or environmental exposures induce protein conformational changes that get passed to offspring, it would create a form of inheritance that looks genetic but isn't. Early-life conditions affecting parents could shape children's health through protein states rather than gene mutations.
This doesn't mean prions explain all missing heritability, but they represent a previously unrecognized layer of biological information that we're only beginning to understand. It's a humbling reminder that our models of inheritance, no matter how sophisticated, are still incomplete.
The discovery of prion-like protein inheritance is more than an academic curiosity. It has immediate, practical implications for medicine, genetics, and how we understand human health.
First, it offers new targets for treating neurodegenerative diseases. If Alzheimer's, Parkinson's, and related conditions involve prion-like propagation of misfolded proteins, therapies that disrupt templating or enhance protein clearance could halt disease progression. Several experimental treatments are already exploring this approach, including antibodies that block protein aggregation and drugs that boost the cellular machinery responsible for degrading misfolded proteins.
Second, it changes how we think about genetic testing and disease risk. If protein states contribute to heritability, sequencing DNA alone won't capture the full picture. Future diagnostic tests might need to assess protein conformations, amyloid load, or other markers of non-genetic inheritance to accurately predict disease risk or explain familial patterns.
Third, it raises questions about inheritance we can't yet answer. If protein states can be inherited, what determines which states get passed down? Can environmental interventions—diet, exercise, stress reduction—alter protein conformations in ways that affect offspring? Could trauma, addiction, or other experiences leave molecular marks in proteins that persist across generations?
The possibility that humans might inherit protein-based traits isn't just speculative. Amyloid-like structures have been observed in human egg cells, though their function remains unknown. Histone modifications, which involve proteins packaging DNA, have been shown to transmit gene expression patterns across generations in mammals. The machinery for protein-based inheritance exists in humans; we just don't know yet how widely it operates or what it does.
Looking ahead, the field is moving fast. Researchers are identifying new prion-forming proteins, mapping how they transmit information, and exploring ways to manipulate them therapeutically. Synthetic biologists are designing artificial prions—proteins engineered to adopt specific conformations and transmit information in controlled ways—that could be used in biosensors, nanomaterials, or even as programmable biological memory systems.
The idea that inheritance operates solely through DNA was always a simplification, a useful model that explained much but not everything. Prion-like protein inheritance shatters that simplification, revealing a hidden layer of biological information that operates in parallel with genes.
Proteins aren't just the products of genetic code; they're information carriers in their own right, capable of storing, copying, and transmitting traits across generations. They can remember, adapt, and evolve in ways DNA cannot, responding to environmental signals and shaping phenotypes without waiting for mutations to accumulate.
This isn't just about adding a footnote to textbooks. It's a paradigm shift that forces us to rethink heredity, evolution, disease, and what it means to inherit. The central dogma—DNA makes RNA makes protein—remains true, but it's no longer the whole story. Proteins make proteins, and in doing so, they write a code of their own.
As Craig P. Hunter, a Harvard professor studying this phenomenon, put it: "In the same way as DNA, amyloids replicate themselves using themselves as a model, which makes them ideal carriers of inheritable information." We're only beginning to understand what that means, but one thing is clear: the future of genetics will be written not just in DNA, but in the folds and shapes of proteins that remember.
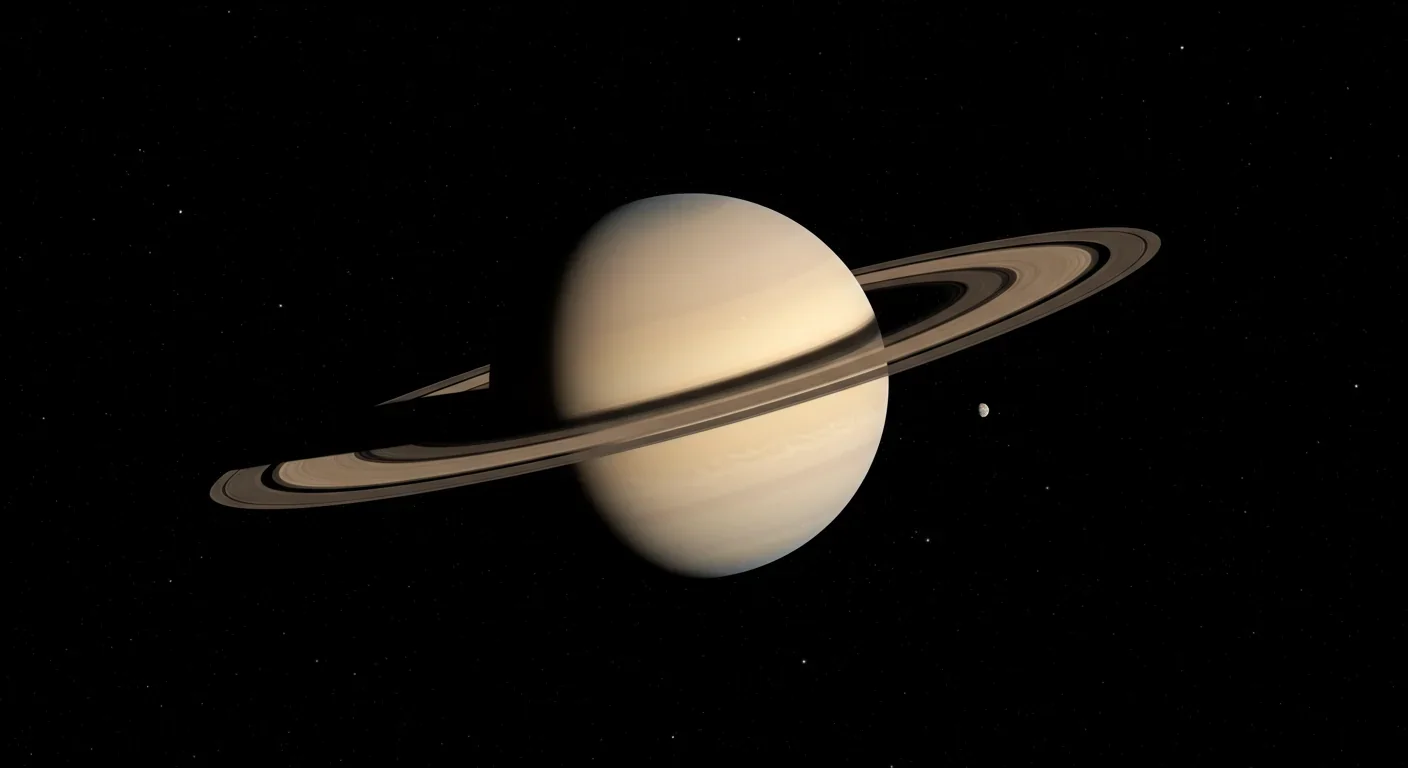
Saturn's iconic rings are temporary, likely formed within the past 100 million years and will vanish in 100-300 million years. NASA's Cassini mission revealed their hidden complexity, ongoing dynamics, and the mysteries that still puzzle scientists.
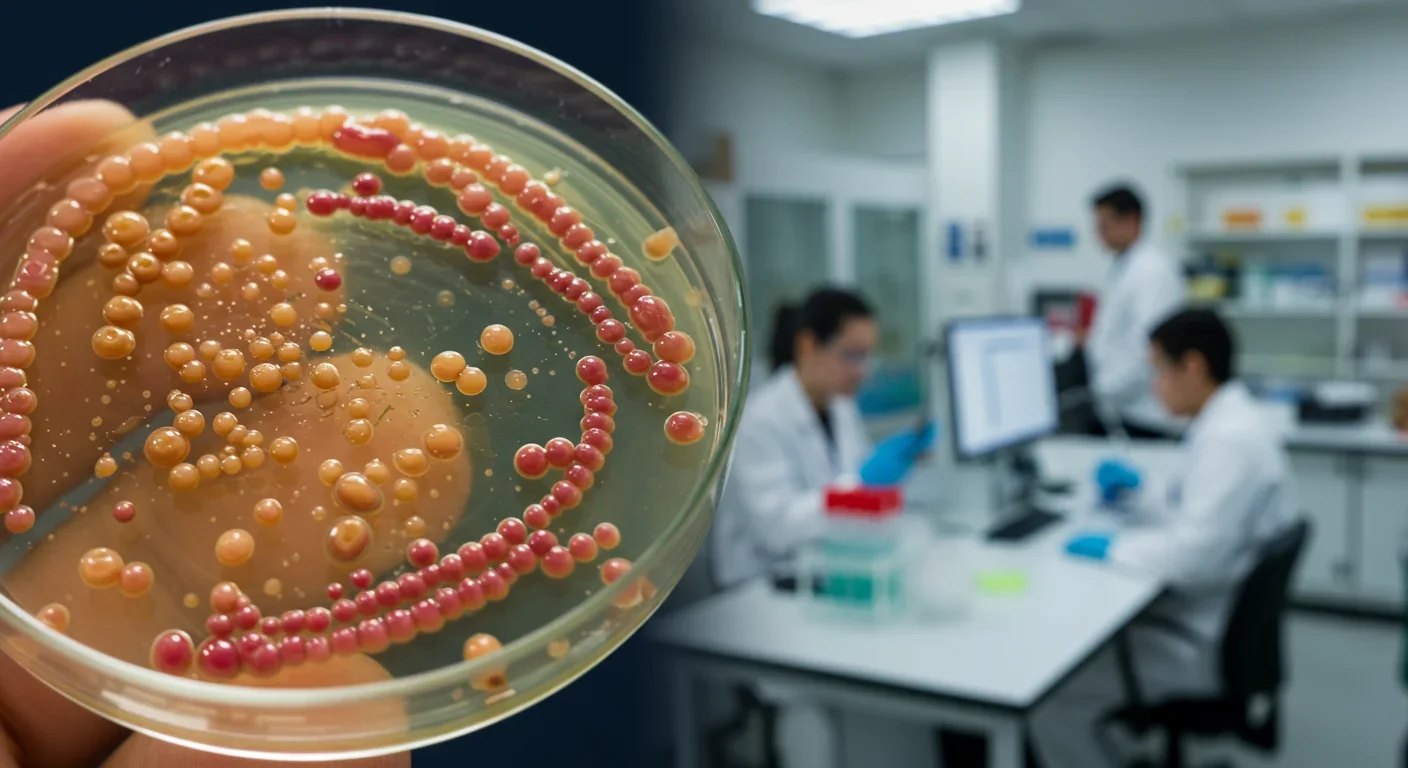
Scientists are revolutionizing gut health by identifying 'keystone' bacteria—crucial microbes that hold entire microbial ecosystems together. By engineering and reintroducing these missing bacterial linchpins, researchers can transform dysfunctional microbiomes into healthy ones, opening new treatments for diseases from IBS to depression.

Marine permaculture—cultivating kelp forests using wave-powered pumps and floating platforms—could sequester carbon 20 times faster than terrestrial forests while creating millions of jobs, feeding coastal communities, and restoring ocean ecosystems. Despite kelp's $500 billion in annual ecosystem services, fewer than 2% of global kelp forests have high-level protection, and over half have vanished in 50 years. Real-world projects in Japan, Chile, the U.S., and Europe demonstrate economic via...

Our attraction to impractical partners stems from evolutionary signals, attachment patterns formed in childhood, and modern status pressures. Understanding these forces helps us make conscious choices aligned with long-term happiness rather than hardwired instincts.

Crows and other corvids bring gifts to humans who feed them, revealing sophisticated social intelligence comparable to primates. This reciprocal exchange behavior demonstrates theory of mind, facial recognition, and long-term memory.

Cryptocurrency has become a revolutionary tool empowering dissidents in authoritarian states to bypass financial surveillance and asset freezes, while simultaneously enabling sanctioned regimes to evade international pressure through parallel financial systems.

Blockchain-based social networks like Bluesky, Mastodon, and Lens Protocol are growing rapidly, offering user data ownership and censorship resistance. While they won't immediately replace Facebook or Twitter, their 51% annual growth rate and new economic models could force Big Tech to fundamentally change how social media works.